Dynamics of virological and immunological markers of HIV persistence after allogeneic haematopoietic stem-cell transplantation in the IciStem cohort: a prospective observational cohort study
- PMID: 38816141
- PMCID: PMC11417461
- DOI: 10.1016/S2352-3018(24)00090-0
Dynamics of virological and immunological markers of HIV persistence after allogeneic haematopoietic stem-cell transplantation in the IciStem cohort: a prospective observational cohort study
Abstract
Background: Allogeneic haematopoietic stem-cell transplantation (allo-HSCT) markedly reduces HIV reservoirs, but the mechanisms by which this occurs are only partly understood. In this study, we aimed to describe the dynamics of virological and immunological markers of HIV persistence after allo-HSCT.
Methods: In this prospective observational cohort study, we analysed the viral reservoir and serological dynamics in IciStem cohort participants with HIV who had undergone allo-HSCT and were receiving antiretroviral therapy, ten of whom had received cells from donors with the CCR5Δ32 mutation. Participants from Belgium, Canada, Germany, Italy, the Netherlands, Spain, Switzerland, and the UK were included in the cohort both prospectively and retrospectively between June 1, 2014 and April 30, 2019. In the first 6 months after allo-HSCT, participants had monthly assessments, with annual assessments thereafter, with the protocol tailored to accommodate for the individual health status of each participant. HIV reservoirs were measured in blood and tissues and HIV-specific antibodies were measured in plasma. We used the Wilcoxon signed-rank test to compare data collected before and after allo-HSCT in participants for whom longitudinal data were available. When the paired test was not possible, we used the Mann-Whitney U test. We developed a mathematical model to study the factors influencing HIV reservoir reduction in people with HIV after allo-HSCT.
Findings: We included 30 people with HIV with haematological malignancies who received a transplant between Sept 1, 2009 and April 30, 2019 and were enrolled within the IciStem cohort and included in this analysis. HIV reservoirs in peripheral blood were reduced immediately after full donor chimerism was achieved, generally accompanied by undetectable HIV-DNA in bone marrow, ileum, lymph nodes, and cerebrospinal fluid, regardless of donor CCR5 genotype. HIV-specific antibody levels and functionality values declined more slowly than direct HIV reservoir values, decaying significantly only months after full donor chimerism. Mathematical modelling suggests that allogeneic immunity mediated by donor cells is the main viral reservoir depletion mechanism after massive reservoir reduction during conditioning chemotherapy before allo-HSCT (half-life of latently infected replication-competent cells decreased from 44 months to 1·5 months).
Interpretation: Our work provides, for the first time, data on the effects of allo-HSCT in the context of HIV infection. Additionally, we raise the question of which marker can serve as the last reporter of the residual viraemia, postulating that the absence of T-cell immune responses might be a more reliable marker than antibody decline after allo-HSCT.
Funding: amfAR (American Foundation for AIDS Research; ARCHE Program), National Institutes of Health, National Institute of Allergy and Infectious Diseases, and Dutch Aidsfonds.
Copyright © 2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests AB reports grants from Gilead Sciences and participating on the advisory board of ViiV Healthcare. AW reports funding for this manuscript from the American Foundation for AIDS Research (amfAR) and Aidsfunds; grants from Gilead and NOW; consulting fees from ViiV Healthcare/GSK, MSD, and Gilead Sciences; participating on the board of the Dutch Federation of Medical Microbiology, the board of the European Society for Translational Antiviral Research, chair on the IAS-USA mutations work group, the Committee of ZonMW (Dutch research organization) Research, and the Committee of the Dutch Federation for Long Covid; and received funding from Ark. AS-C reports funding for this manuscript from amfAR; grants from ANRS, the National Institutes of Health (NIH), Institute Pasteur, and MSDAVENIR; honoraria from MSD, ViiV Healthcare, and Gilead Sciences; and is chair of the Scientific and Medical Committee of Sidaction. B-EOJ reports consulting fees from Gilead Sciences, ViiV Healthcare, and Merck Sharp & Dohme; honoraria from Gilead Sciences and ViiV Healthcare; travel expenses for attending meetings from Gilead; and is scientific secretary for the German AIDS Society. BR reports honoraria from Gilead Sciences, Janssen, and ViiV Healthcare; payment for advice from ViiV Healthcare; and travel expenses for attending meetings and travel from ViiV Healthcare and Gilead Sciences. GH reports travel expenses for attending the meeting and travel for the HIV Persistence Workshop 2022. JB reports receiving honoraria from AbbVie, Pfizer, and Gilead Sciences; and travel expenses for attending meetings from AbbVie, Pfizer, and Gilead Sciences. JK reports grants from Novartis and Miltenyi Biotech; royalties from GADETA and Miltenyi Biotech; a patent with GADETA; and holds stock interest in GADETA. JM-P reports funding for this manuscript from amfAR. JSZW reports funding for this manuscript from The German Center for Infection Research, EU H2020 Research and Innovation Programme, HW & J Hector Foundation, the German Research Foundation, The Hamburg Investment and Development Bank, and amfAR; and honoraria from Nobite, GSK, and Gilead Sciences. JTS reports funding for this manuscript from the NIH and National Institute of Allergy and Infectious Diseases. LB report grants from Abbvie and Gilead Sciences; consulting fees from Abbvie and Gilead Sciences; and honoraria from AbbVie and Gilead Sciences. LV reports receiving grants from ViiV Healthcare and Gilead Sciences; and consulting fees from ViiV Healthcare and Gilead Sciences. MJG and GA declare being an employee of Ragon Institute of Mass General, MIT, and Harvard during the study; and an employee of Moderna afterwards. MNi reports receiving consulting fees from Gilead Sciences; and honoraria for lectures from ViiV Healthcare. All other authors declare no competing interests.
Figures


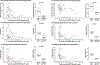

References
-
- Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999; 5: 512–7. - PubMed
-
- Hütter G, Nowak D, Mossner M, et al. Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation. New England Journal of Medicine 2009; 360: 692–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

