Human enteric nervous system progenitor transplantation improves functional responses in Hirschsprung disease patient-derived tissue
- PMID: 38816188
- PMCID: PMC11347211
- DOI: 10.1136/gutjnl-2023-331532
Human enteric nervous system progenitor transplantation improves functional responses in Hirschsprung disease patient-derived tissue
Abstract
Objective: Hirschsprung disease (HSCR) is a severe congenital disorder affecting 1:5000 live births. HSCR results from the failure of enteric nervous system (ENS) progenitors to fully colonise the gastrointestinal tract during embryonic development. This leads to aganglionosis in the distal bowel, resulting in disrupted motor activity and impaired peristalsis. Currently, the only viable treatment option is surgical resection of the aganglionic bowel. However, patients frequently suffer debilitating, lifelong symptoms, with multiple surgical procedures often necessary. Hence, alternative treatment options are crucial. An attractive strategy involves the transplantation of ENS progenitors generated from human pluripotent stem cells (hPSCs).
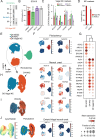
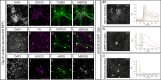
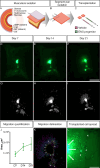
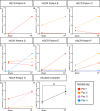
Design: ENS progenitors were generated from hPSCs using an accelerated protocol and characterised, in detail, through a combination of single-cell RNA sequencing, protein expression analysis and calcium imaging. We tested ENS progenitors' capacity to integrate and affect functional responses in HSCR colon, after ex vivo transplantation to organotypically cultured patient-derived colonic tissue, using organ bath contractility.
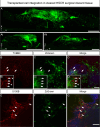
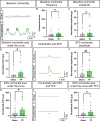
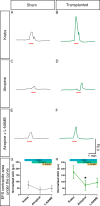
Results: We found that our protocol consistently gives rise to high yields of a cell population exhibiting transcriptional and functional hallmarks of early ENS progenitors. Following transplantation, hPSC-derived ENS progenitors integrate, migrate and form neurons/glia within explanted human HSCR colon samples. Importantly, the transplanted HSCR tissue displayed significantly increased basal contractile activity and increased responses to electrical stimulation compared with control tissue.
Conclusion: Our findings demonstrate, for the first time, the potential of hPSC-derived ENS progenitors to repopulate and increase functional responses in human HSCR patient colonic tissue.
Keywords: ENTERIC NERVOUS SYSTEM; GASTROINTESTINAL SURGERY; HIRSCHSPRUNG'S DISEASE; STEM CELLS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
