Kidney outcomes of SGLT2 inhibitors among older patients with diabetic kidney disease in real-world clinical practice: the Japan Chronic Kidney Disease Database Ex
- PMID: 38816204
- PMCID: PMC11141184
- DOI: 10.1136/bmjdrc-2024-004115
Kidney outcomes of SGLT2 inhibitors among older patients with diabetic kidney disease in real-world clinical practice: the Japan Chronic Kidney Disease Database Ex
Abstract
Introduction: We compared the kidney outcomes between patients with diabetic kidney disease (DKD) aged ≥75 years initiating sodium-glucose cotransporter 2 (SGLT2) inhibitors versus other glucose-lowering drugs, additionally presenting with or without proteinuria.
Research design and methods: Using the Japan Chronic Kidney Disease Database, we developed propensity scores, implementing a 1:1 matching protocol. The primary outcome included the decline rate in estimated glomerular filtration rate (eGFR), and secondary outcomes incorporated a composite of a 40% reduction in eGFR or progression to end-stage kidney disease.
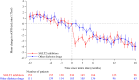
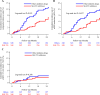
Results: At baseline, the mean age at initiation of SGLT2 inhibitors (n=348) or other glucose-lowering medications (n=348) was 77.7 years. The mean eGFR was 59.3 mL/min/1.73m2 and proteinuria was 230 (33.0%) patients. Throughout the follow-up period, the mean annual rate of eGFR change was -0.80 mL/min/1.73 m2/year (95% CI -1.05 to -0.54) among SGLT2 inhibitors group and -1.78 mL/min/1.73 m2/year (95% CI -2.08 to -1.49) in other glucose-lowering drugs group (difference in the rate of eGFR decline between the groups was 0.99 mL/min/1.73 m2/year (95% CI 0.5 to 1.38)), favoring SGLT2 inhibitors (p<0.001). Composite renal outcomes were observed 38 in the SGLT2 inhibitors group and 57 in the other glucose-lowering medications group (HR 0.64, 95% CI 0.42 to 0.97). There was no evidence of an interaction between SGLT2 inhibitors initiation and proteinuria.
Conclusions: The benefits of SGLT2 inhibitors on renal outcomes are also applicable to older patients with DKD aged≥75 years.
Keywords: Diabetes Mellitus, Type 2.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: YY received research support from AstraZeneca, Bayer, and Daiichi Sankyo and lecture fee from Otsuka Pharmaceutical and Torii Pharmaceutical. NK received lecture fee from AstraZeneca, Otsuka Pharmaceutical, Novartis, Daiichi Sankyo, Takeda Pharmaceutical, Kyowa Kirin, Mitsubishi Tanabe Pharma, Astellas Pharma, and scholarship donations from Astellas Pharma, Teijin Pharma, AstraZeneca, Kyowa Kirin, Bayer Yakuhin, Chugai Pharmaceutical, Otsuka Pharmaceutical, Nippon Boehringer Ingelheim, Daiichi Sankyo, Takeda Pharmaceutical Company Limited, additionally, funded by Daiichi Sankyo, AstraZeneca, Bayer, and Nobelpharma. KT received research support from AstraZeneca, Ono, Bayer, Kyowa Kirin, Otsuka Pharmaceutical, Takeda Pharmaceutical, and Daiichi Sankyo and received lecture fee from Novartis, AstraZeneca, Ono, Daiichi Sankyo, Takeda Pharmaceutical, Otsuka Pharmaceutical, Bayer, and Kyowa Kirin. NC and HA are employees of AstraZeneca.
Figures


Similar articles
-
Kidney Outcomes Associated With SGLT2 Inhibitors Versus Other Glucose-Lowering Drugs in Real-world Clinical Practice: The Japan Chronic Kidney Disease Database.Diabetes Care. 2021 Nov;44(11):2542-2551. doi: 10.2337/dc21-1081. Epub 2021 Sep 30. Diabetes Care. 2021. PMID: 34593566 Free PMC article.
-
Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study.Lancet Diabetes Endocrinol. 2020 Jan;8(1):27-35. doi: 10.1016/S2213-8587(19)30384-5. Lancet Diabetes Endocrinol. 2020. PMID: 31862149
-
Factors affecting the sodium-glucose cotransporter 2 inhibitors-related initial decline in glomerular filtration rate and its possible effect on kidney outcome in chronic kidney disease with type 2 diabetes: The Japan Chronic Kidney Disease Database.Diabetes Obes Metab. 2024 Jul;26(7):2905-2914. doi: 10.1111/dom.15611. Epub 2024 May 8. Diabetes Obes Metab. 2024. PMID: 38719436
-
Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis.Diabetes Obes Metab. 2019 May;21(5):1237-1250. doi: 10.1111/dom.13648. Epub 2019 Mar 4. Diabetes Obes Metab. 2019. PMID: 30697905
-
Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease.Curr Diab Rep. 2022 Jan;22(1):39-52. doi: 10.1007/s11892-021-01442-z. Epub 2022 Feb 3. Curr Diab Rep. 2022. PMID: 35113333 Free PMC article. Review.
Cited by
-
Long-term preservation of kidney function with SGLT-2 inhibitors versus comparator drugs in people with type 2 diabetes and chronic kidney disease.Diabetes Obes Metab. 2025 Sep;27(9):5182-5191. doi: 10.1111/dom.16569. Epub 2025 Jul 2. Diabetes Obes Metab. 2025. PMID: 40600449 Free PMC article.
-
Emerging New Era of Artificial Intelligence and Digital Medicine-directed Management of Chronic Kidney Disease.JMA J. 2025 Jan 15;8(1):57-59. doi: 10.31662/jmaj.2024-0221. Epub 2024 Sep 20. JMA J. 2025. PMID: 39926059 Free PMC article. No abstract available.
-
Dual-faced guardians: SGLT2 inhibitors' kidney protection and health challenges: a position statement by Kasralainy nephrology group (KANG).Diabetol Metab Syndr. 2025 Jun 14;17(1):214. doi: 10.1186/s13098-025-01790-w. Diabetol Metab Syndr. 2025. PMID: 40517274 Free PMC article. Review.
-
An Observational Study of Evidence-Based Therapies in Older Patients with Heart Failure with Reduced Ejection Fraction: Insights from a Dedicated Heart Failure Clinic.J Clin Med. 2024 Nov 26;13(23):7171. doi: 10.3390/jcm13237171. J Clin Med. 2024. PMID: 39685630 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
