Neoantigen-specific stimulation of tumor-infiltrating lymphocytes enables effective TCR isolation and expansion while preserving stem-like memory phenotypes
- PMID: 38816232
- PMCID: PMC11141192
- DOI: 10.1136/jitc-2023-008645
Neoantigen-specific stimulation of tumor-infiltrating lymphocytes enables effective TCR isolation and expansion while preserving stem-like memory phenotypes
Abstract
Background: Tumor-infiltrating lymphocytes (TILs) targeting neoantigens can effectively treat a selected set of metastatic solid cancers. However, harnessing TILs for cancer treatments remains challenging because neoantigen-reactive T cells are often rare and exhausted, and ex vivo expansion can further reduce their frequencies. This complicates the identification of neoantigen-reactive T-cell receptors (TCRs) and the development of TIL products with high reactivity for patient treatment.
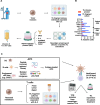
Methods: We tested whether TILs could be in vitro stimulated against neoantigens to achieve selective expansion of neoantigen-reactive TILs. Given their prevalence, mutant p53 or RAS were studied as models of human neoantigens. An in vitro stimulation method, termed "NeoExpand", was developed to provide neoantigen-specific stimulation to TILs. 25 consecutive patient TILs from tumors harboring p53 or RAS mutations were subjected to NeoExpand.
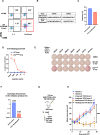
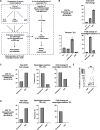
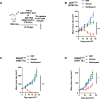
Results: We show that neoantigenic stimulation achieved selective expansion of neoantigen-reactive TILs and broadened the neoantigen-reactive CD4+ and CD8+ TIL clonal repertoire. This allowed the effective isolation of novel neoantigen-reactive TCRs. Out of the 25 consecutive TIL samples, neoantigenic stimulation enabled the identification of 16 unique reactivities and 42 TCRs, while conventional TIL expansion identified 9 reactivities and 14 TCRs. Single-cell transcriptome analysis revealed that neoantigenic stimulation increased neoantigen-reactive TILs with stem-like memory phenotypes expressing IL-7R, CD62L, and KLF2. Furthermore, neoantigenic stimulation improved the in vivo antitumor efficacy of TILs relative to the conventional OKT3-induced rapid TIL expansion in p53-mutated or KRAS-mutated xenograft mouse models.
Conclusions: Taken together, neoantigenic stimulation of TILs selectively expands neoantigen-reactive TILs by frequencies and by their clonal repertoire. NeoExpand led to improved phenotypes and functions of neoantigen-reactive TILs. Our data warrant its clinical evaluation.
Trial registration number: NCT00068003, NCT01174121, and NCT03412877.
Keywords: Adoptive cell therapy - ACT; T cell Receptor - TCR; Tumor infiltrating lymphocyte - TIL.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NL, SPK and SAR have a pending patent application. The rest of the authors report no competing interest.
Figures


References
-
- Parkhurst MR, Robbins PF, Tran E, et al. . Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discovery 2019;9:1022–35. 10.1158/2159-8290.CD-18-1494 - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
