Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression
- PMID: 38816352
- PMCID: PMC11139893
- DOI: 10.1038/s41467-024-48441-8
Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression
Abstract
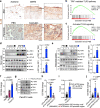
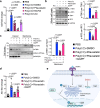
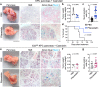
Chronic inflammation is a major cause of cancer worldwide. Interleukin 33 (IL-33) is a critical initiator of cancer-prone chronic inflammation; however, its induction mechanism by environmental causes of chronic inflammation is unknown. Herein, we demonstrate that Toll-like receptor (TLR)3/4-TBK1-IRF3 pathway activation links environmental insults to IL-33 induction in the skin and pancreas inflammation. An FDA-approved drug library screen identifies pitavastatin to effectively suppress IL-33 expression by blocking TBK1 membrane recruitment/activation through the mevalonate pathway inhibition. Accordingly, pitavastatin prevents chronic pancreatitis and its cancer sequela in an IL-33-dependent manner. The IRF3-IL-33 axis is highly active in chronic pancreatitis and its associated pancreatic cancer in humans. Interestingly, pitavastatin use correlates with a significantly reduced risk of chronic pancreatitis and pancreatic cancer in patients. Our findings demonstrate that blocking the TBK1-IRF3-IL-33 signaling axis suppresses cancer-prone chronic inflammation. Statins present a safe and effective prophylactic strategy to prevent chronic inflammation and its cancer sequela.
© 2024. The Author(s).
Conflict of interest statement
J.H.P. and S.D. are coinventors on a filed patent for the use of IL-33 inhibition in treating cancer, fibrosis, and inflammation (PCT/US21/40725). A.M. is an equity holder of DermBiont Inc. The remaining authors state no conflict of interest.
Figures



Update of
-
Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression.Res Sq [Preprint]. 2023 Jan 12:rs.3.rs-2318750. doi: 10.21203/rs.3.rs-2318750/v1. Res Sq. 2023. Update in: Nat Commun. 2024 May 30;15(1):4099. doi: 10.1038/s41467-024-48441-8. PMID: 36711701 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- CAMS/Burroughs Wellcome Fund (BWF)
- P01 CA117969/CA/NCI NIH HHS/United States
- K08 AR068619/AR/NIAMS NIH HHS/United States
- R01 CA219670/CA/NCI NIH HHS/United States
- R01 CA215498/CA/NCI NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
- K08AR068619/U.S. Department of Health & Human Services | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- T32 GM144273/GM/NIGMS NIH HHS/United States
- R01 AR076013/AR/NIAMS NIH HHS/United States
- R01AR076013/U.S. Department of Health & Human Services | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- Scholar/Sidney Kimmel Foundation
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

