Bioprinting salivary gland models and their regenerative applications
- PMID: 38816372
- PMCID: PMC11139920
- DOI: 10.1038/s41405-024-00219-2
Bioprinting salivary gland models and their regenerative applications
Abstract
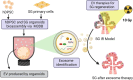
Objective: Salivary gland (SG) hypofunction is a common clinical condition arising from radiotherapy to suppress head and neck cancers. The radiation often destroys the SG secretory acini, and glands are left with limited regenerative potential. Due to the complex architecture of SG acini and ducts, three-dimensional (3D) bioprinting platforms have emerged to spatially define these in vitro epithelial units and develop mini-organs or organoids for regeneration. Due to the limited body of evidence, this comprehensive review highlights the advantages and challenges of bioprinting platforms for SG regeneration.
Methods: SG microtissue engineering strategies such as magnetic 3D bioassembly of cells and microfluidic coaxial 3D bioprinting of cell-laden microfibers and microtubes have been proposed to replace the damaged acinar units, avoid the use of xenogeneic matrices (like Matrigel), and restore salivary flow.
Results: Replacing the SG damaged organ is challenging due to its complex architecture, which combines a ductal network with acinar epithelial units to facilitate a unidirectional flow of saliva. Our research group was the first to develop 3D bioassembly SG epithelial functional organoids with innervation to respond to both cholinergic and adrenergic stimulation. More recently, microtissue engineering using coaxial 3D bioprinting of hydrogel microfibers and microtubes could also supported the formation of viable epithelial units. Both bioprinting approaches could overcome the need for Matrigel by facilitating the assembly of adult stem cells, such as human dental pulp stem cells, and primary SG cells into micro-sized 3D constructs able to produce their own matrix and self-organize into micro-modular tissue clusters with lumenized areas. Furthermore, extracellular vesicle (EV) therapies from organoid-derived secretome were also designed and validated ex vivo for SG regeneration after radiation damage.
Conclusion: Magnetic 3D bioassembly and microfluidic coaxial bioprinting platforms have the potential to create SG mini-organs for regenerative applications via organoid transplantation or organoid-derived EV therapies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Ethical approval and consent were not applicable since this manuscript is a narrative review of the existing literature.
Figures




Similar articles
-
Trends in Salivary Gland Tissue Engineering: From Stem Cells to Secretome and Organoid Bioprinting.Tissue Eng Part B Rev. 2021 Apr;27(2):155-165. doi: 10.1089/ten.TEB.2020.0149. Epub 2020 Aug 26. Tissue Eng Part B Rev. 2021. PMID: 32723016 Review.
-
Salivary gland regeneration: from salivary gland stem cells to three-dimensional bioprinting.SLAS Technol. 2023 Jun;28(3):199-209. doi: 10.1016/j.slast.2023.03.004. Epub 2023 Apr 3. SLAS Technol. 2023. PMID: 37019217
-
Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands.Biomaterials. 2018 Oct;180:52-66. doi: 10.1016/j.biomaterials.2018.06.011. Epub 2018 Jun 12. Biomaterials. 2018. PMID: 30025245
-
A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids.J Tissue Eng Regen Med. 2019 Mar;13(3):495-508. doi: 10.1002/term.2809. Epub 2019 Mar 6. J Tissue Eng Regen Med. 2019. PMID: 30666813
-
Three-Dimensional Bioprinting Nanotechnologies towards Clinical Application of Stem Cells and Their Secretome in Salivary Gland Regeneration.Stem Cells Int. 2016;2016:7564689. doi: 10.1155/2016/7564689. Epub 2016 Dec 20. Stem Cells Int. 2016. PMID: 28090208 Free PMC article. Review.
Cited by
-
Tuning the Supramolecular Polymerization and Cell Response of Ureidopyrimidinone Monomers by Pushing the Hydrophobic Threshold.J Am Chem Soc. 2025 Jun 25;147(25):21478-21491. doi: 10.1021/jacs.5c01445. Epub 2025 Jun 11. J Am Chem Soc. 2025. PMID: 40498553 Free PMC article.
-
Revolutionising oral organoids with artificial intelligence.Biomater Transl. 2024 Nov 15;5(4):372-389. doi: 10.12336/biomatertransl.2024.04.004. eCollection 2024. Biomater Transl. 2024. PMID: 39872928 Free PMC article. Review.
-
Poly (hydroxyethyl methacrylate) Saliva-Gel: A Polymer-Based Solution for Xerostomia Treatment.ACS Appl Polym Mater. 2025 Jul 17:10.1021/acsapm.5c00881. doi: 10.1021/acsapm.5c00881. Online ahead of print. ACS Appl Polym Mater. 2025. PMID: 40687500 Free PMC article.
-
Role of Exosomes in Salivary Gland Tumors and Technological Advances in Their Assessment.Cancers (Basel). 2024 Sep 27;16(19):3298. doi: 10.3390/cancers16193298. Cancers (Basel). 2024. PMID: 39409917 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

