The link between ancient microbial fluoride resistance mechanisms and bioengineering organofluorine degradation or synthesis
- PMID: 38816380
- PMCID: PMC11139923
- DOI: 10.1038/s41467-024-49018-1
The link between ancient microbial fluoride resistance mechanisms and bioengineering organofluorine degradation or synthesis
Abstract
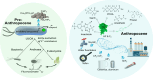
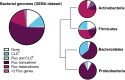
Fluorinated organic chemicals, such as per- and polyfluorinated alkyl substances (PFAS) and fluorinated pesticides, are both broadly useful and unusually long-lived. To combat problems related to the accumulation of these compounds, microbial PFAS and organofluorine degradation and biosynthesis of less-fluorinated replacement chemicals are under intense study. Both efforts are undermined by the substantial toxicity of fluoride, an anion that powerfully inhibits metabolism. Microorganisms have contended with environmental mineral fluoride over evolutionary time, evolving a suite of detoxification mechanisms. In this perspective, we synthesize emerging ideas on microbial defluorination/fluorination and fluoride resistance mechanisms and identify best approaches for bioengineering new approaches for degrading and making organofluorine compounds.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A prescription for engineering PFAS biodegradation.Biochem J. 2024 Dec 4;481(23):1757-1770. doi: 10.1042/BCJ20240283. Biochem J. 2024. PMID: 39585294 Free PMC article. Review.
-
Microwell fluoride assay screening for enzymatic defluorination.Methods Enzymol. 2024;696:65-83. doi: 10.1016/bs.mie.2023.12.020. Epub 2024 Jan 11. Methods Enzymol. 2024. PMID: 38658089
-
Microwell Fluoride Screen for Chemical, Enzymatic, and Cellular Reactions Reveals Latent Microbial Defluorination Capacity for -CF3 Groups.Appl Environ Microbiol. 2022 May 10;88(9):e0028822. doi: 10.1128/aem.00288-22. Epub 2022 Apr 18. Appl Environ Microbiol. 2022. PMID: 35435713 Free PMC article.
-
Recombinant Pseudomonas growing on non-natural fluorinated substrates shows stress but overall tolerance to cytoplasmically released fluoride anion.mBio. 2024 Jan 16;15(1):e0278523. doi: 10.1128/mbio.02785-23. Epub 2023 Dec 8. mBio. 2024. PMID: 38063407 Free PMC article.
-
Nothing lasts forever: understanding microbial biodegradation of polyfluorinated compounds and perfluorinated alkyl substances.Microb Biotechnol. 2022 Mar;15(3):773-792. doi: 10.1111/1751-7915.13928. Epub 2021 Sep 27. Microb Biotechnol. 2022. PMID: 34570953 Free PMC article. Review.
Cited by
-
Engineering Inorganic Pyrophosphate Metabolism as a Strategy to Generate a Fluoride-Resistant Saccharomyces cerevisiae Strain.Microorganisms. 2025 Jan 21;13(2):226. doi: 10.3390/microorganisms13020226. Microorganisms. 2025. PMID: 40005593 Free PMC article.
-
PFAS Biodegradation and the Constraints of Thermodynamics.Microb Biotechnol. 2025 Jun;18(6):e70181. doi: 10.1111/1751-7915.70181. Microb Biotechnol. 2025. PMID: 40524434 Free PMC article.
-
A prescription for engineering PFAS biodegradation.Biochem J. 2024 Dec 4;481(23):1757-1770. doi: 10.1042/BCJ20240283. Biochem J. 2024. PMID: 39585294 Free PMC article. Review.
-
Enzymatic carbon-fluorine bond cleavage by human gut microbes.Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2504122122. doi: 10.1073/pnas.2504122122. Epub 2025 Jun 13. Proc Natl Acad Sci U S A. 2025. PMID: 40512801 Free PMC article.
-
Enhancement of Fluoride's Antibacterial and Antibiofilm Effects against Oral Staphylococcus aureus by the Urea Derivative BPU.Antibiotics (Basel). 2024 Sep 30;13(10):930. doi: 10.3390/antibiotics13100930. Antibiotics (Basel). 2024. PMID: 39452197 Free PMC article.
References
-
- Weeks ME. The discovery of the elements. XVII. The halogen family. J. Chem. Educ. 1932;9:1915–1938. doi: 10.1021/ed009p1915. - DOI
-
- Gordin MD. Facing the music: how original was Borodin’s chemistry? J. Chem. Educ. 2006;83:561. doi: 10.1021/ed083p561. - DOI
-
- Barnabas SJ, et al. Extraction of chemical structures from literature and patent documents using open access chemistry toolkits: a case study with PFAS. Digit. Discov. 2022;1:490–501. doi: 10.1039/D2DD00019A. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

