Lumbar instability remodels cartilage endplate to induce intervertebral disc degeneration by recruiting osteoclasts via Hippo-CCL3 signaling
- PMID: 38816384
- PMCID: PMC11139958
- DOI: 10.1038/s41413-024-00331-x
Lumbar instability remodels cartilage endplate to induce intervertebral disc degeneration by recruiting osteoclasts via Hippo-CCL3 signaling
Abstract
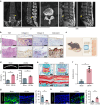
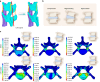
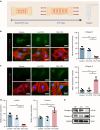
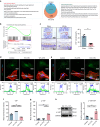
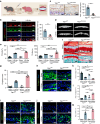
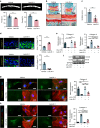
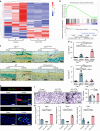
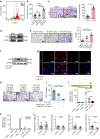
Degenerated endplate appears with cheese-like morphology and sensory innervation, contributing to low back pain and subsequently inducing intervertebral disc degeneration in the aged population.1 However, the origin and development mechanism of the cheese-like morphology remain unclear. Here in this study, we report lumbar instability induced cartilage endplate remodeling is responsible for this pathological change. Transcriptome sequencing of the endplate chondrocytes under abnormal stress revealed that the Hippo signaling was key for this process. Activation of Hippo signaling or knockout of the key gene Yap1 in the cartilage endplate severed the cheese-like morphological change and disc degeneration after lumbar spine instability (LSI) surgery, while blocking the Hippo signaling reversed this process. Meanwhile, transcriptome sequencing data also showed osteoclast differentiation related gene set expression was up regulated in the endplate chondrocytes under abnormal mechanical stress, which was activated after the Hippo signaling. Among the discovered osteoclast differentiation gene set, CCL3 was found to be largely released from the chondrocytes under abnormal stress, which functioned to recruit and promote osteoclasts formation for cartilage endplate remodeling. Over-expression of Yap1 inhibited CCL3 transcription by blocking its promoter, which then reversed the endplate from remodeling to the cheese-like morphology. Finally, LSI-induced cartilage endplate remodeling was successfully rescued by local injection of an AAV5 wrapped Yap1 over-expression plasmid at the site. These findings suggest that the Hippo signaling induced osteoclast gene set activation in the cartilage endplate is a potential new target for the management of instability induced low back pain and lumbar degeneration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
N6-Methyladenosine-Modified circSMAD4 Prevents Lumbar Instability Induced Cartilage Endplate Ossification.Adv Sci (Weinh). 2025 Apr;12(13):e2413970. doi: 10.1002/advs.202413970. Epub 2025 Feb 12. Adv Sci (Weinh). 2025. PMID: 39936497 Free PMC article.
-
Osteoporosis of the vertebra and osteochondral remodeling of the endplate causes intervertebral disc degeneration in ovariectomized mice.Arthritis Res Ther. 2018 Sep 10;20(1):207. doi: 10.1186/s13075-018-1701-1. Arthritis Res Ther. 2018. PMID: 30201052 Free PMC article.
-
Chlorogenic Acid retards cartilaginous endplate degeneration and ameliorates intervertebral disc degeneration via suppressing NF-κB signaling.Life Sci. 2021 Jun 1;274:119324. doi: 10.1016/j.lfs.2021.119324. Epub 2021 Mar 9. Life Sci. 2021. PMID: 33711382
-
Emerging role and function of Hippo-YAP/TAZ signaling pathway in musculoskeletal disorders.Stem Cell Res Ther. 2024 Oct 29;15(1):386. doi: 10.1186/s13287-024-04011-9. Stem Cell Res Ther. 2024. PMID: 39468616 Free PMC article. Review.
-
Research Progress on the Role of Cartilage Endplate in Intervertebral Disc Degeneration.Cell Biochem Funct. 2024 Sep;42(7):e4118. doi: 10.1002/cbf.4118. Cell Biochem Funct. 2024. PMID: 39267363 Review.
Cited by
-
In-silico analysis predicts disruption of normal angiogenesis as a causative factor in osteoporosis pathogenesis.BMC Genom Data. 2024 Oct 8;25(1):85. doi: 10.1186/s12863-024-01269-z. BMC Genom Data. 2024. PMID: 39379846 Free PMC article.
-
Targeting advanced glycation end products: potential therapeutic approaches for mitigating diabetic intervertebral disc degeneration?Front Endocrinol (Lausanne). 2025 Jul 7;16:1618984. doi: 10.3389/fendo.2025.1618984. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40692599 Free PMC article. Review.
-
Cellular senescence and other age-related mechanisms in skeletal diseases.Bone Res. 2025 Jul 7;13(1):68. doi: 10.1038/s41413-025-00448-7. Bone Res. 2025. PMID: 40623977 Free PMC article. Review.
-
The Hippo pathway in bone and cartilage: implications for development and disease.PeerJ. 2025 Apr 22;13:e19334. doi: 10.7717/peerj.19334. eCollection 2025. PeerJ. 2025. PMID: 40292098 Free PMC article. Review.
-
N6-Methyladenosine-Modified circSMAD4 Prevents Lumbar Instability Induced Cartilage Endplate Ossification.Adv Sci (Weinh). 2025 Apr;12(13):e2413970. doi: 10.1002/advs.202413970. Epub 2025 Feb 12. Adv Sci (Weinh). 2025. PMID: 39936497 Free PMC article.
References
-
- Centers for Disease, C. & Prevention Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morb. Mortal. Wkly Rep. 58, 421–426 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

