Capillary wave tweezer
- PMID: 38816398
- PMCID: PMC11637047
- DOI: 10.1038/s41598-024-63154-0
Capillary wave tweezer
Abstract
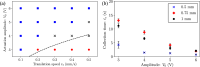
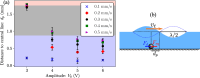
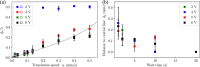
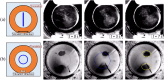
Precise control of microparticle movement is crucial in high throughput processing for various applications in scalable manufacturing, such as particle monolayer assembly and 3D bio-printing. Current techniques using acoustic, electrical and optical methods offer precise manipulation advantages, but their scalability is restricted due to issues such as, high input powers and complex fabrication and operation processes. In this work, we introduce the concept of capillary wave tweezers, where mm-scale capillary wave fields are dynamically manipulated to control the position of microparticles in a liquid volume. Capillary waves are generated in an open liquid volume using low frequency vibrations (in the range of 10-100 Hz) to trap particles underneath the nodes of the capillary waves. By shifting the displacement nodes of the waves, the trapped particles are precisely displaced. Using analytical and numerical models, we identify conditions under which a stable control over particle motion is achieved. By showcasing the ability to dynamically control the movement of microparticles, our concept offers a simple and high throughput method to manipulate particles in open systems.
Keywords: Acoustic; Capillary; Microparticles; Streaming; Vibration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ashkin, A. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett.24, 156–159. 10.1103/PhysRevLett.24.156 (1970).
-
- Ashkin, A., Dziedzic, J. M., Bjorkholm, J. E. & Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett.11, 288–290. 10.1364/OL.11.000288 (1986). - PubMed
-
- Cheng, K., Guo, J., Fu, Y. & Guo, J. Active microparticle manipulation: Recent advances. Sens. Actuat. A322, 112616. 10.1016/j.sna.2021.112616 (2021).
-
- Shundo, A., Hori, K., Penaloza, J., David, P. & Tanaka, K. Optical tweezers with fluorescence detection for temperature-dependent microrheological measurements. Rev. Sci. Instrum.84, 014103. 10.1063/1.4789429 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

