Maternal behaviours disrupted by Gprasp2 deletion modulate neurodevelopmental trajectory in progeny
- PMID: 38816497
- PMCID: PMC11139669
- DOI: 10.1038/s41598-024-62088-x
Maternal behaviours disrupted by Gprasp2 deletion modulate neurodevelopmental trajectory in progeny
Abstract
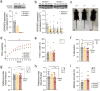
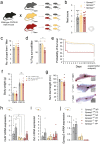
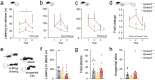
Autism spectrum disorders (ASDs) are known to present sex-specific differences. At the same time, understanding how maternal behaviours are affected by pathogenic mutations is crucial to translate research efforts since rearing may recursively modulate neurodevelopment phenotype of the progeny. In this work, we focused on the effects of Gprasp2 deletion in females and its impact in progeny care and development. Female mice, wild-type (WT), Gprasp2+/- (HET) or Gprasp2-/- (KO) mutants and their progeny were used and behavioural paradigms targeting anxiety, memory, maternal care, and other social behaviours were performed. Analysis of communication was carried out through daily recordings of ultrasonic vocalizations in isolated pups and cross-fostering experiments were performed to understand the effect of maternal genotype in pup development. We found that Gprasp2-/- females presented striking impairments in social and working memory. Females also showed disruptions in maternal care, as well as physiological and molecular alterations in the reproductive system and hypothalamus, such as the structure of the mammary gland and the expression levels of oxytocin receptor (OxtR) in nulliparous versus primiparous females. We observed alterations in pup communication, particularly a reduced number of calls in Gprasp2 KO pups, which resulted from an interaction effect of the dam and pup genotype. Cross-fostering mutant pups with wild-type dams rescued some of the early defects shown in vocalizations, however, this effect was not bidirectional, as rearing WT pups with Gprasp2-/- dams was not sufficient to induce significant phenotypical alterations. Our results suggest Gprasp2 mutations perturb social and working memory in a sex-independent manner, but impact female-specific behaviours towards progeny care, female physiology, and gene expression. These changes in mutant dams contribute to a disruption in early stages of progeny development. More generally, our results highlight the need to better understand GxE interactions in the context of ASDs, when female behaviour may present a contributing factor in postnatal neurodevelopmental trajectory.
Keywords: Autism; Cross fostering; GPRASP2; Maternal care; Oxytocin receptor; Sex differences; Ultrasonic vocalisations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Endocannabinoid receptor deficiency affects maternal care and alters the dam's hippocampal oxytocin receptor and brain-derived neurotrophic factor expression.J Neuroendocrinol. 2013 Oct;25(10):898-909. doi: 10.1111/jne.12082. J Neuroendocrinol. 2013. PMID: 23895426
-
Abnormal mGluR-mediated synaptic plasticity and autism-like behaviours in Gprasp2 mutant mice.Nat Commun. 2019 Mar 29;10(1):1431. doi: 10.1038/s41467-019-09382-9. Nat Commun. 2019. PMID: 30926797 Free PMC article.
-
Dopamine receptor D2 deficiency reduces mouse pup ultrasonic vocalizations and maternal responsiveness.Genes Brain Behav. 2013 Jun;12(4):397-404. doi: 10.1111/gbb.12037. Epub 2013 Apr 15. Genes Brain Behav. 2013. PMID: 23521753
-
Oxytocin signal and social behaviour: comparison among adult and infant oxytocin, oxytocin receptor and CD38 gene knockout mice.J Neuroendocrinol. 2010 May;22(5):373-9. doi: 10.1111/j.1365-2826.2010.01976.x. Epub 2010 Jan 5. J Neuroendocrinol. 2010. PMID: 20141571 Review.
-
New aspects of oxytocin receptor function revealed by knockout mice: sociosexual behaviour and control of energy balance.Prog Brain Res. 2008;170:79-90. doi: 10.1016/S0079-6123(08)00408-1. Prog Brain Res. 2008. PMID: 18655874 Review.
References
-
- Association AP. Diagnostic and statistical manual of mental disorders 5th ed 2013.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

