Spectrum of MRI findings in central nervous system affection in Lyme neuroborreliosis
- PMID: 38816506
- PMCID: PMC11139962
- DOI: 10.1038/s41598-024-63006-x
Spectrum of MRI findings in central nervous system affection in Lyme neuroborreliosis
Abstract
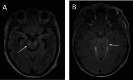
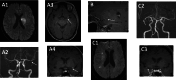
Affections of the central nervous system (CNS) rarely occur in Lyme neuroborreliosis (LNB). CNS manifestations can have residual neurological symptoms despite antibiotic treatment. We explored the spectrum of CNS affections in patients with LNB in a tertiary care center in a region endemic for Lyme borreliosis. We retrospectively included patients treated at a tertiary care center from January 2020-December 2021 fulfilling the case criteria for LNB as stated in the current German guideline on LNB. Clinical data, cerebrospinal fluid (CSF) findings and MRI imaging were collected. We included 35 patients with LNB, 24 with early manifestations and 11 with CNS-LNB. CNS-LNB patients had encephalomyelitis (n = 6) or cerebral vasculitis (n = 5). Patients with early LNB and CNS-LNB differed regarding albumin CSF/serum quotient and total protein in CSF. Duration from onset of symptoms until diagnosis was statistically significantly longer in patients with encephalomyelitis. MRI findings were heterogeneous and showed longitudinal extensive myelitis, perimedullar leptomeningeal enhancement, pontomesencephalic lesions or cerebral vasculitis. CNS-LNB can present with a variety of clinical syndromes and MRI changes. No clear pattern of MRI findings in CNS-LNB could be identified. The role of MRI consists in ruling out other causes of neurological symptoms.
Keywords: CSF; Cerebral MRI imaging; Encephalitis; Encephalomyelitis; Lyme disease; Lyme neuroborreliosis; Myelitis; Neuroinfectious diseases; Vasculitis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

