Structural basis for the bi-specificity of USP25 and USP28 inhibitors
- PMID: 38816515
- PMCID: PMC11239673
- DOI: 10.1038/s44319-024-00167-w
Structural basis for the bi-specificity of USP25 and USP28 inhibitors
Abstract
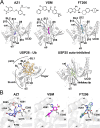
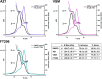
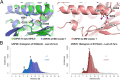
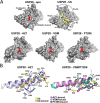
The development of cancer therapeutics is often hindered by the fact that specific oncogenes cannot be directly pharmaceutically addressed. Targeting deubiquitylases that stabilize these oncogenes provides a promising alternative. USP28 and USP25 have been identified as such target deubiquitylases, and several small-molecule inhibitors indiscriminately inhibiting both enzymes have been developed. To obtain insights into their mode of inhibition, we structurally and functionally characterized USP28 in the presence of the three different inhibitors AZ1, Vismodegib and FT206. The compounds bind into a common pocket acting as a molecular sink. Our analysis provides an explanation why the two enzymes are inhibited with similar potency while other deubiquitylases are not affected. Furthermore, a key glutamate residue at position 366/373 in USP28/USP25 plays a central structural role for pocket stability and thereby for inhibition and activity. Obstructing the inhibitor-binding pocket by mutation of this glutamate may provide a tool to accelerate future drug development efforts for selective inhibitors of either USP28 or USP25 targeting distinct binding pockets.
Keywords: Cancer; DUB; Inhibitor; USP25; USP28.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Altun, Kramer M, Willems HB, McDermott LI, Leach JL, Goldenberg CA, Kumar KGS SJ, Konietzny R, Fischer R, Kogan E, et al. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem Biol. 2011;18:1401–1412. doi: 10.1016/j.chembiol.2011.08.018. - DOI - PubMed
-
- Báez-Santos YM, Barraza SJ, Wilson MW, Agius MP, Mielech AM, Davis NM, Baker SC, Larsen SD, Mesecar AD. X-ray structural and biological evaluation of a series of potent and highly selective inhibitors of human coronavirus papain-like proteases. J Med Chem. 2014;57:2393–2412. doi: 10.1021/jm401712t. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

