SGLT2 inhibition eliminates senescent cells and alleviates pathological aging
- PMID: 38816549
- PMCID: PMC11257941
- DOI: 10.1038/s43587-024-00642-y
SGLT2 inhibition eliminates senescent cells and alleviates pathological aging
Abstract
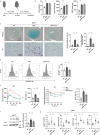
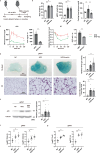
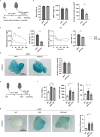
It has been reported that accumulation of senescent cells in various tissues contributes to pathological aging and that elimination of senescent cells (senolysis) improves age-associated pathologies. Here, we demonstrate that inhibition of sodium-glucose co-transporter 2 (SGLT2) enhances clearance of senescent cells, thereby ameliorating age-associated phenotypic changes. In a mouse model of dietary obesity, short-term treatment with the SGLT2 inhibitor canagliflozin reduced the senescence load in visceral adipose tissue and improved adipose tissue inflammation and metabolic dysfunction, but normalization of plasma glucose by insulin treatment had no effect on senescent cells. Canagliflozin extended the lifespan of mice with premature aging even when treatment was started in middle age. Metabolomic analyses revealed that short-term treatment with canagliflozin upregulated 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, enhancing immune-mediated clearance of senescent cells by downregulating expression of programmed cell death-ligand 1. These findings suggest that inhibition of SGLT2 has an indirect senolytic effect by enhancing endogenous immunosurveillance of senescent cells.
© 2024. The Author(s).
Conflict of interest statement
T.M. discloses research funds and remuneration for lectures from Mitsubishi Tanabe Pharma Corporation. Canagliflozin was provided by Mitsubishi Tanabe Pharma Corporation. The funder of the study had no role in the design and conduct of the study, the collection, management, analysis and interpretation of the data, the preparation, review or approval of the manuscript, and the decision to submit the manuscript for publication. The other authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
- 23H05487/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 22K18389/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 19K07316/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- JP20gm1110012/Japan Agency for Medical Research and Development (AMED)
- 21zf0127003s0201/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Medical

