Aging atlas reveals cell-type-specific effects of pro-longevity strategies
- PMID: 38816550
- PMCID: PMC11257944
- DOI: 10.1038/s43587-024-00631-1
Aging atlas reveals cell-type-specific effects of pro-longevity strategies
Abstract
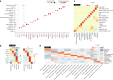
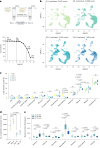
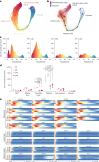
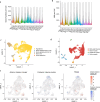
Organismal aging involves functional declines in both somatic and reproductive tissues. Multiple strategies have been discovered to extend lifespan across species. However, how age-related molecular changes differ among various tissues and how those lifespan-extending strategies slow tissue aging in distinct manners remain unclear. Here we generated the transcriptomic Cell Atlas of Worm Aging (CAWA, http://mengwanglab.org/atlas ) of wild-type and long-lived strains. We discovered cell-specific, age-related molecular and functional signatures across all somatic and germ cell types. We developed transcriptomic aging clocks for different tissues and quantitatively determined how three different pro-longevity strategies slow tissue aging distinctively. Furthermore, through genome-wide profiling of alternative polyadenylation (APA) events in different tissues, we discovered cell-type-specific APA changes during aging and revealed how these changes are differentially affected by the pro-longevity strategies. Together, this study offers fundamental molecular insights into both somatic and reproductive aging and provides a valuable resource for in-depth understanding of the diversity of pro-longevity mechanisms.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Update of
-
Aging Atlas Reveals Cell-Type-Specific Regulation of Pro-longevity Strategies.bioRxiv [Preprint]. 2023 Mar 18:2023.02.28.530490. doi: 10.1101/2023.02.28.530490. bioRxiv. 2023. Update in: Nat Aging. 2024 Jul;4(7):998-1013. doi: 10.1038/s43587-024-00631-1. PMID: 36909655 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
- P20 GM139762/GM/NIGMS NIH HHS/United States
- P01 AG066606/AG/NIA NIH HHS/United States
- P40 OD010440/OD/NIH HHS/United States
- R00 AG062746/AG/NIA NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- DHHS NE-LB606/Nebraska Department of Health and Human Services (Nebraska DHHS)
- R01AG062257/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- RF1 AG062257/AG/NIA NIH HHS/United States
- R00AG062746/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- DP1 DK113644/DK/NIDDK NIH HHS/United States
- DP1DK113644/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P20GM139762/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P01AG066606/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- DHHS NE-LB595/Nebraska Department of Health and Human Services (Nebraska DHHS)
- R01 AG045183/AG/NIA NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- R01 AT009050/AT/NCCIH NIH HHS/United States
- RR200063/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

