Beyond the serotonin deficit hypothesis: communicating a neuroplasticity framework of major depressive disorder
- PMID: 38816586
- PMCID: PMC11692567
- DOI: 10.1038/s41380-024-02625-2
Beyond the serotonin deficit hypothesis: communicating a neuroplasticity framework of major depressive disorder
Abstract
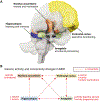
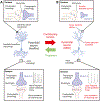
The serotonin deficit hypothesis explanation for major depressive disorder (MDD) has persisted among clinicians and the general public alike despite insufficient supporting evidence. To combat rising mental health crises and eroding public trust in science and medicine, researchers and clinicians must be able to communicate to patients and the public an updated framework of MDD: one that is (1) accessible to a general audience, (2) accurately integrates current evidence about the efficacy of conventional serotonergic antidepressants with broader and deeper understandings of pathophysiology and treatment, and (3) capable of accommodating new evidence. In this article, we summarize a framework for the pathophysiology and treatment of MDD that is informed by clinical and preclinical research in psychiatry and neuroscience. First, we discuss how MDD can be understood as inflexibility in cognitive and emotional brain circuits that involves a persistent negativity bias. Second, we discuss how effective treatments for MDD enhance mechanisms of neuroplasticity-including via serotonergic interventions-to restore synaptic, network, and behavioral function in ways that facilitate adaptive cognitive and emotional processing. These treatments include typical monoaminergic antidepressants, novel antidepressants like ketamine and psychedelics, and psychotherapy and neuromodulation techniques. At the end of the article, we discuss this framework from the perspective of effective science communication and provide useful language and metaphors for researchers, clinicians, and other professionals discussing MDD with a general or patient audience.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: CNE receives consulting feeds from Sage Therapeutics and Health Rhythms for Research unrelated to this manuscript. CNE participates on scientific advisory boards for Embark/Neuro and BabyScripts, and has unpaid roles with the National Network of Depression Centers (Member at Large), The Society of Biological Psychiatry (Council Member), and the American College of Neuropsychopharmacology (Executive Council). SMT is listed as an inventor on and receives royalties from patents related to treating depression that are held by the University of Maryland, Baltimore. SMT has received honoraria from several non-profit or academic institutions and from companies developing psychiatric drugs for general lectures and advice about depression treatments. SMT has patents issued and pending from the University of Maryland, Baltimore, for various means for treating depression. SMT serves as an unpaid member of several committees for the American College of Neuropsychopharmacology. AMN is supported by the National Institute of Child Health and Development K23HD110435. CEP and KAD have no disclosures.
Figures


References
-
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–22. - PubMed
-
- Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;113:1237–64. - PubMed
-
- France CM, Lysaker PH, Robinson RP. The ‘Chemical Imbalance’ explanation for depression: origins, lay endorsement, and clinical implications. Prof Psychol Res Pr. 2007;38:411–20.
-
- Owens MJ. Selectivity of antidepressants: from the monoamine hypothesis of depression to the SSRI revolution and beyond. J Clin Psychiatry. 2004;65:5–10. - PubMed
-
- Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, Heninger GR, et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry. 1996;53:117–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

