Signaling pathways in liver cancer: pathogenesis and targeted therapy
- PMID: 38816668
- PMCID: PMC11139849
- DOI: 10.1186/s43556-024-00184-0
Signaling pathways in liver cancer: pathogenesis and targeted therapy
Abstract
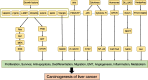
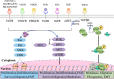
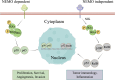
Liver cancer remains one of the most prevalent malignancies worldwide with high incidence and mortality rates. Due to its subtle onset, liver cancer is commonly diagnosed at a late stage when surgical interventions are no longer feasible. This situation highlights the critical role of systemic treatments, including targeted therapies, in bettering patient outcomes. Despite numerous studies on the mechanisms underlying liver cancer, tyrosine kinase inhibitors (TKIs) are the only widely used clinical inhibitors, represented by sorafenib, whose clinical application is greatly limited by the phenomenon of drug resistance. Here we show an in-depth discussion of the signaling pathways frequently implicated in liver cancer pathogenesis and the inhibitors targeting these pathways under investigation or already in use in the management of advanced liver cancer. We elucidate the oncogenic roles of these pathways in liver cancer especially hepatocellular carcinoma (HCC), as well as the current state of research on inhibitors respectively. Given that TKIs represent the sole class of targeted therapeutics for liver cancer employed in clinical practice, we have particularly focused on TKIs and the mechanisms of the commonly encountered phenomena of its resistance during HCC treatment. This necessitates the imperative development of innovative targeted strategies and the urgency of overcoming the existing limitations. This review endeavors to shed light on the utilization of targeted therapy in advanced liver cancer, with a vision to improve the unsatisfactory prognostic outlook for those patients.
Keywords: Hepatocellular carcinoma (HCC); Liver cancer; Signaling pathways; Sorafenib; Targeted therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma.World J Gastroenterol. 2019 Aug 7;25(29):3870-3896. doi: 10.3748/wjg.v25.i29.3870. World J Gastroenterol. 2019. PMID: 31413525 Free PMC article. Review.
-
Dual inhibition of Akt and c-Met as a second-line therapy following acquired resistance to sorafenib in hepatocellular carcinoma cells.Mol Oncol. 2017 Mar;11(3):320-334. doi: 10.1002/1878-0261.12039. Epub 2017 Feb 17. Mol Oncol. 2017. PMID: 28164434 Free PMC article.
-
Molecular targeted therapy for hepatocellular carcinoma: current and future.World J Gastroenterol. 2013 Oct 7;19(37):6144-55. doi: 10.3748/wjg.v19.i37.6144. World J Gastroenterol. 2013. PMID: 24115810 Free PMC article. Review.
-
Targeted therapies in hepatocellular carcinoma.Curr Med Chem. 2014;21(8):966-74. doi: 10.2174/09298673113209990234. Curr Med Chem. 2014. PMID: 23992323 Review.
-
Targeting tyrosine kinase receptors in hepatocellular carcinoma.Curr Cancer Drug Targets. 2013 Mar;13(3):300-12. doi: 10.2174/15680096113139990075. Curr Cancer Drug Targets. 2013. PMID: 23016985 Review.
Cited by
-
Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review).Int J Mol Med. 2025 Jul;56(1):104. doi: 10.3892/ijmm.2025.5545. Epub 2025 May 9. Int J Mol Med. 2025. PMID: 40342021 Free PMC article. Review.
-
Hippo/YAP signaling's multifaceted crosstalk in cancer.Front Cell Dev Biol. 2025 Jul 2;13:1595362. doi: 10.3389/fcell.2025.1595362. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40673277 Free PMC article. Review.
-
Donafenib activates the p53 signaling pathway in hepatocellular carcinoma, induces ferroptosis, and enhances cell apoptosis.Clin Exp Med. 2025 Jan 3;25(1):29. doi: 10.1007/s10238-024-01550-6. Clin Exp Med. 2025. PMID: 39753901 Free PMC article.
-
Mapping the knowledge domains of literature on hepatocellular carcinoma and liver failure: a bibliometric approach.Front Oncol. 2025 Apr 16;15:1529297. doi: 10.3389/fonc.2025.1529297. eCollection 2025. Front Oncol. 2025. PMID: 40308492 Free PMC article.
-
NRF1-Induced lncRNA DDX11-AS1 Contributes to the Progression of Hepatocellular Carcinoma via Activating CA9 Expression and the MEK/ERK Pathway.J Hepatocell Carcinoma. 2025 May 7;12:891-908. doi: 10.2147/JHC.S516656. eCollection 2025. J Hepatocell Carcinoma. 2025. PMID: 40356690 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
