Placental growth factor promotes neural invasion and predicts disease prognosis in resectable pancreatic cancer
- PMID: 38816706
- PMCID: PMC11138065
- DOI: 10.1186/s13046-024-03066-z
Placental growth factor promotes neural invasion and predicts disease prognosis in resectable pancreatic cancer
Abstract
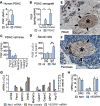
Background: Surgery represents the only curative treatment option for pancreatic ductal adenocarcinoma (PDAC), but recurrence in more than 85% of patients limits the success of curative-intent tumor resection. Neural invasion (NI), particularly the spread of tumor cells along nerves into extratumoral regions of the pancreas, constitutes a well-recognized risk factor for recurrence. Hence, monitoring and therapeutic targeting of NI offer the potential to stratify recurrence risk and improve recurrence-free survival. Based on the evolutionary conserved dual function of axon and vessel guidance molecules, we hypothesize that the proangiogenic vessel guidance factor placental growth factor (PlGF) fosters NI. To test this hypothesis, we correlated PlGF with NI in PDAC patient samples and functionally assessed its role for the interaction of tumor cells with nerves.
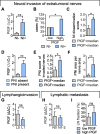
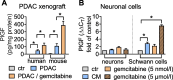
Methods: Serum levels of PlGF and its soluble receptor sFlt1, and expression of PlGF mRNA transcripts in tumor tissues were determined by ELISA or qPCR in a retrospective discovery and a prospective validation cohort. Free circulating PlGF was calculated from the ratio PlGF/sFlt1. Incidence and extent of NI were quantified based on histomorphometric measurements and separately assessed for intratumoral and extratumoral nerves. PlGF function on reciprocal chemoattraction and directed neurite outgrowth was evaluated in co-cultures of PDAC cells with primary dorsal-root-ganglia neurons or Schwann cells using blocking anti-PlGF antibodies.
Results: Elevated circulating levels of free PlGF correlated with NI and shorter overall survival in patients with PDAC qualifying for curative-intent surgery. Furthermore, high tissue PlGF mRNA transcript levels in patients undergoing curative-intent surgery correlated with a higher incidence and greater extent of NI spreading to tumor-distant extratumoral nerves. In turn, more abundant extratumoral NI predicted shorter disease-free and overall survival. Experimentally, PlGF facilitated directional and dynamic changes in neurite outgrowth of primary dorsal-root-ganglia neurons upon exposure to PDAC derived guidance and growth factors and supported mutual chemoattraction of tumor cells with neurons and Schwann cells.
Conclusion: Our translational results highlight PlGF as an axon guidance factor, which fosters neurite outgrowth and attracts tumor cells towards nerves. Hence, PlGF represents a promising circulating biomarker of NI and potential therapeutic target to improve the clinical outcome for patients with resectable PDAC.
Keywords: Axon guidance molecule; Cancer neuroscience; Nerves; Neural invasion; Pancreatic cancer; Placental growth factor (PlGF).
© 2024. The Author(s).
Conflict of interest statement
CF has a patent pending related to the content of this work. All other authors disclose no potential conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

