Boosting antitumor efficacy using docetaxel-loaded nanoplatforms: from cancer therapy to regenerative medicine approaches
- PMID: 38816723
- PMCID: PMC11137998
- DOI: 10.1186/s12967-024-05347-9
Boosting antitumor efficacy using docetaxel-loaded nanoplatforms: from cancer therapy to regenerative medicine approaches
Abstract
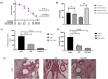
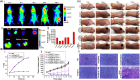
The intersection of nanotechnology and pharmacology has revolutionized the delivery and efficacy of chemotherapeutic agents, notably docetaxel, a key drug in cancer treatment. Traditionally limited by poor solubility and significant side effects, docetaxel's therapeutic potential has been significantly enhanced through its incorporation into nanoplatforms, such as nanofibers and nanoparticles. This advancement offers targeted delivery, controlled release, and improved bioavailability, dramatically reducing systemic toxicity and enhancing patient outcomes. Nanofibers provide a versatile scaffold for the controlled release of docetaxel, utilizing techniques like electrospinning to tailor drug release profiles. Nanoparticles, on the other hand, enable precise drug delivery to tumor cells, minimizing damage to healthy tissues through sophisticated encapsulation methods such as nanoprecipitation and emulsion. These nanotechnologies not only improve the pharmacokinetic properties of docetaxel but also open new avenues in regenerative medicine by facilitating targeted therapy and cellular regeneration. This narrative review highlights the transformative impact of docetaxel-loaded nanoplatforms in oncology and beyond, showcasing the potential of nanotechnology to overcome the limitations of traditional chemotherapy and pave the way for future innovations in drug delivery and regenerative therapies. Through these advancements, nanotechnology promises a new era of precision medicine, enhancing the efficacy of cancer treatments while minimizing adverse effects.
Keywords: Antitumor activity; Docetaxel-loaded nanoplatforms; Regenerative medicine; Tissue engineering.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures













Similar articles
-
Docetaxel-loaded PAMAM-based poly (γ-benzyl-l-glutamate)-b-d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles in human breast cancer and human cervical cancer therapy.J Microencapsul. 2019 Sep;36(6):552-565. doi: 10.1080/02652048.2019.1654002. J Microencapsul. 2019. PMID: 31403342
-
Novel T7-Modified pH-Responsive Targeted Nanosystem for Co-Delivery of Docetaxel and Curcumin in the Treatment of Esophageal Cancer.Int J Nanomedicine. 2020 Oct 9;15:7745-7762. doi: 10.2147/IJN.S257312. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33116498 Free PMC article.
-
In Vitro and In Vivo Evaluation of Novel DTX-Loaded Multifunctional Heparin-Based Polymeric Micelles Targeting Folate Receptors and Endosomes.Recent Pat Anticancer Drug Discov. 2020;15(4):341-359. doi: 10.2174/1574892815666201006124604. Recent Pat Anticancer Drug Discov. 2020. PMID: 33023456
-
Nanotechnology-Enhanced siRNA Delivery: Revolutionizing Cancer Therapy.ACS Appl Bio Mater. 2025 Jun 16;8(6):4549-4579. doi: 10.1021/acsabm.5c00489. Epub 2025 May 12. ACS Appl Bio Mater. 2025. PMID: 40354673 Review.
-
Advancements in Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Breast Cancer Therapy.Curr Pharm Des. 2024;30(37):2922-2936. doi: 10.2174/0113816128319233240725103706. Curr Pharm Des. 2024. PMID: 39150028 Review.
Cited by
-
Recent advances in hydrogels applications for tissue engineering and clinical trials.Regen Ther. 2024 Aug 28;26:635-645. doi: 10.1016/j.reth.2024.08.015. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39281106 Free PMC article. Review.
-
Nanomedicine for cancer patient-centered care.MedComm (2020). 2024 Oct 20;5(11):e767. doi: 10.1002/mco2.767. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39434967 Free PMC article. Review.
References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clinrnal. 2024;74(1):12–49. - PubMed
-
- Malvezzi M, Santucci C, Boffetta P, Collatuzzo G, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2023 with focus on lung cancer. Ann Oncol. 2023;34(4):410–419. - PubMed
-
- Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today. 2010;15(19–20):842–850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

