Enhancing reporting through structure: a before and after study on the effectiveness of SPIRIT-based templates to improve the completeness of reporting of randomized controlled trial protocols
- PMID: 38816752
- PMCID: PMC11140857
- DOI: 10.1186/s41073-024-00147-7
Enhancing reporting through structure: a before and after study on the effectiveness of SPIRIT-based templates to improve the completeness of reporting of randomized controlled trial protocols
Abstract
Background: Despite the improvements in the completeness of reporting of randomized trial protocols after the publication of the Standard Protocol Items: Recommendations for Interventional Trial (SPIRIT) guidelines, many items remain poorly reported. This study aimed to assess the effectiveness of using SPIRIT-tailored templates for trial protocols to improve the completeness of reporting of the protocols that master's students write as part of their master's theses.
Methods: Before and after experimental study performed at the University Master's Degree in Orthopaedic Manual Physiotherapy of the Universitat Internacional de Catalunya (Barcelona, Spain). While students in the post-intervention period were instructed to use a trial protocol template that was tailored to SPIRIT, students in the pre-intervention period did not use the template.
Primary outcome: Difference between the pre- and post-intervention periods in the mean number of adequately reported items (0-10 scale). The outcomes were evaluated independently and in duplicate by two blinded assessors. Students and their supervisors were not aware that they were part of a research project. For the statistical analysis, we used a generalized linear regression model (dependent variable: number of adequately reported items in the protocol; independent variables: intervention period, call, language).
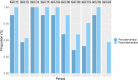
Results: Thirty-four trial protocols were included (17, pre-intervention; 17, post-intervention). Protocols produced during the post-intervention period (mean: 8.24; SD: 1.52) were more completely reported than those produced during the pre-intervention period (mean: 6.35; SD: 1.80); adjusted difference: 1.79 (95% CI: 0.58 to 3.00).
Conclusions: SPIRIT-based templates could be used to improve the completeness of reporting of randomized trial protocols.
Keywords: Clinical trial protocol; Meta-research; Randomized controlled trial; Reporting guidelines; Reporting quality.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Speich B, Mann E, Schönenberger CM, Mellor K, Griessbach AN, Dhiman P, et al. Reminding Peer Reviewers of Reporting Guideline Items to Improve Completeness in Published Articles: Primary Results of 2 Randomized Trials. JAMA Netw Open. 2023;6(6):e2317651–e2317651. doi: 10.1001/jamanetworkopen.2023.17651. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous