M1 macrophage-derived exosomes promote intervertebral disc degeneration by enhancing nucleus pulposus cell senescence through LCN2/NF-κB signaling axis
- PMID: 38816771
- PMCID: PMC11140985
- DOI: 10.1186/s12951-024-02556-8
M1 macrophage-derived exosomes promote intervertebral disc degeneration by enhancing nucleus pulposus cell senescence through LCN2/NF-κB signaling axis
Abstract
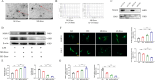
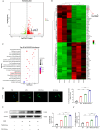
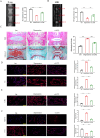
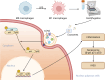
Intervertebral disc degeneration (IVDD) is the primary factor contributing to low back pain (LBP). Unlike elderly patients, many young IVDD patients usually have a history of trauma or long-term abnormal stress, which may lead to local inflammatory reaction causing by immune cells, and ultimately accelerates degeneration. Research has shown the significance of M1-type macrophages in IVDD; nevertheless, the precise mechanism and the route by which it influences the function of nucleus pulposus cell (NPC) remain unknown. Utilizing a rat acupuncture IVDD model and an NPC degeneration model induced by lipopolysaccharide (LPS), we investigated the function of M1 macrophage-derived exosomes (M1-Exos) in IVDD both in vivo and in vitro in this study. We found that M1-Exos enhanced LPS-induced NPC senescence, increased the number of SA-β-gal-positive cells, blocked the cell cycle, and promoted the activation of P21 and P53. M1-Exos derived from supernatant pretreated with the exosome inhibitor GW4869 reversed this result in vivo and in vitro. RNA-seq showed that Lipocalin2 (LCN2) was enriched in M1-Exos and targeted the NF-κB pathway. The quantity of SA-β-gal-positive cells was significantly reduced with the inhibition of LCN2, and the expression of P21 and P53 in NPCs was decreased. The same results were obtained in the acupuncture-induced IVDD model. In addition, inhibition of LCN2 promotes the expression of type II collagen (Col-2) and inhibits the expression of matrix metalloproteinase 13 (MMP13), thereby restoring the equilibrium of metabolism inside the extracellular matrix (ECM) in vitro and in vivo. In addition, the NF-κB pathway is crucial for regulating M1-Exo-mediated NPC senescence. After the addition of M1-Exos to LPS-treated NPCs, p-p65 activity was significantly activated, while si-LCN2 treatment significantly inhibited p-p65 activity. Therefore, this paper demonstrates that M1 macrophage-derived exosomes have the ability to deliver LCN2, which activates the NF-κB signaling pathway, and exacerbates IVDD by accelerating NPC senescence. This may shed new light on the mechanism of IVDD and bring a fresh approach to IVDD therapy.
Keywords: Cellular senescence; Exosome; Intervertebral disc degeneration; LCN2; Macrophage.
© 2024. The Author(s).
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures




Similar articles
-
FSTL1 Accelerates Nucleus Pulposus Cell Senescence and Intervertebral Disc Degeneration Through TLR4/NF-κB Pathway.Inflammation. 2024 Aug;47(4):1229-1247. doi: 10.1007/s10753-024-01972-0. Epub 2024 Feb 6. Inflammation. 2024. PMID: 38316670
-
Inhibition of MAGL attenuates Intervertebral Disc Degeneration by Delaying nucleus pulposus senescence through STING.Int Immunopharmacol. 2024 Apr 20;131:111904. doi: 10.1016/j.intimp.2024.111904. Epub 2024 Mar 22. Int Immunopharmacol. 2024. PMID: 38518595
-
Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy.Stem Cells. 2021 Apr;39(4):467-481. doi: 10.1002/stem.3322. Epub 2021 Jan 18. Stem Cells. 2021. PMID: 33459443 Free PMC article.
-
Cellular senescence - Molecular mechanisms of intervertebral disc degeneration from an immune perspective.Biomed Pharmacother. 2023 Jun;162:114711. doi: 10.1016/j.biopha.2023.114711. Epub 2023 Apr 20. Biomed Pharmacother. 2023. PMID: 37084562 Review.
-
Mechanistic Interactions Driving Nucleus Pulposus Cell Senescence in Intervertebral Disc Degeneration: A Multi-Axial Perspective of Mechanical, Immune, and Metabolic Pathways.JOR Spine. 2025 Jul 2;8(3):e70089. doi: 10.1002/jsp2.70089. eCollection 2025 Sep. JOR Spine. 2025. PMID: 40606198 Free PMC article. Review.
Cited by
-
Chaperone-mediated autophagy directs a dual mechanism to balance premature senescence and senolysis to prevent intervertebral disc degeneration.Bone Res. 2025 Jun 12;13(1):62. doi: 10.1038/s41413-025-00441-0. Bone Res. 2025. PMID: 40506462 Free PMC article.
-
Integrated bulk and single-cell RNA sequencing to identify potential biomarkers in intervertebral disc degeneration.Eur J Med Res. 2025 Feb 14;30(1):102. doi: 10.1186/s40001-025-02346-4. Eur J Med Res. 2025. PMID: 39953636 Free PMC article.
-
Targeting FAP-positive chondrocytes in osteoarthritis: a novel lipid nanoparticle siRNA approach to mitigate cartilage degeneration.J Nanobiotechnology. 2024 Oct 26;22(1):659. doi: 10.1186/s12951-024-02946-y. J Nanobiotechnology. 2024. PMID: 39456041 Free PMC article.
-
LT-α Facilitates the Aerobic Glycolysis and M1 Polarization of Macrophages by Activating the NF-κB Signaling Pathway in Intervertebral Disc Degeneration.J Inflamm Res. 2025 Mar 19;18:4103-4120. doi: 10.2147/JIR.S506162. eCollection 2025. J Inflamm Res. 2025. PMID: 40125079 Free PMC article.
-
Role of macrophage in intervertebral disc degeneration.Bone Res. 2025 Jan 23;13(1):15. doi: 10.1038/s41413-024-00397-7. Bone Res. 2025. PMID: 39848963 Free PMC article. Review.
References
-
- Carapetis JR, Dadi AF. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global burden of disease study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
-
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

