Bridging the gap between in vitro and in vivo models: a way forward to clinical translation of mitochondrial transplantation in acute disease states
- PMID: 38816774
- PMCID: PMC11140916
- DOI: 10.1186/s13287-024-03771-8
Bridging the gap between in vitro and in vivo models: a way forward to clinical translation of mitochondrial transplantation in acute disease states
Abstract
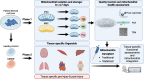
Mitochondrial transplantation and transfer are being explored as therapeutic options in acute and chronic diseases to restore cellular function in injured tissues. To limit potential immune responses and rejection of donor mitochondria, current clinical applications have focused on delivery of autologous mitochondria. We recently convened a Mitochondrial Transplant Convergent Working Group (CWG), to explore three key issues that limit clinical translation: (1) storage of mitochondria, (2) biomaterials to enhance mitochondrial uptake, and (3) dynamic models to mimic the complex recipient tissue environment. In this review, we present a summary of CWG conclusions related to these three issues and provide an overview of pre-clinical studies aimed at building a more robust toolkit for translational trials.
Keywords: Ischemia–reperfusion injury; Joint-on-a-chip; Mitochondria; Mitochondrial transplant; Organ-on-a-chip.
© 2024. The Author(s).
Conflict of interest statement
SEH and TPJ are paid employees of United Therapeutics. LA and MM are paid employees of the Centre for Commercialization of Regenerative Medicine. MR and YZ are inventors on a patent describing Biowire II heart-on-a-chip technology that is licensed to Valo Health. They receive royalty payments. ACA is the founder and scientific director for MITO2i. No author has or will receive financial incentive of any kind in relation to the technology described herein.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

