TGF-β signaling regulates differentiation of MSCs in bone metabolism: disputes among viewpoints
- PMID: 38816830
- PMCID: PMC11140988
- DOI: 10.1186/s13287-024-03761-w
TGF-β signaling regulates differentiation of MSCs in bone metabolism: disputes among viewpoints
Abstract
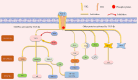
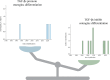
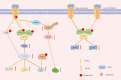
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into cells of different lineages to form mesenchymal tissues, which are promising in regard to treatment for bone diseases. Their osteogenic differentiation is under the tight regulation of intrinsic and extrinsic factors. Transforming growth factor β (TGF-β) is an essential growth factor in bone metabolism, which regulates the differentiation of MSCs. However, published studies differ in their views on whether TGF-β signaling regulates the osteogenic differentiation of MSCs positively or negatively. The controversial results have not been summarized systematically and the related explanations are required. Therefore, we reviewed the basics of TGF-β signaling and summarized how each of three isoforms regulates osteogenic differentiation. Three isoforms of TGF-β (TGF-β1/β2/β3) play distinct roles in regulating osteogenic differentiation of MSCs. Additionally, other possible sources of conflicts are summarized here. Further understanding of TGF-β signaling regulation in MSCs may lead to new applications to promote bone regeneration and improve therapies for bone diseases.
Keywords: Bone metabolism; MSCs; Osteogenic differentiation; TGF-β.
© 2024. The Author(s).
Conflict of interest statement
The authors declared that they have no competing interests.
Figures

Similar articles
-
rhBMP-2 induces terminal differentiation of human bone marrow mesenchymal stromal cells only by synergizing with other signals.Stem Cell Res Ther. 2024 Apr 29;15(1):124. doi: 10.1186/s13287-024-03735-y. Stem Cell Res Ther. 2024. PMID: 38679735 Free PMC article.
-
Galunisertib downregulates mutant type I collagen expression and promotes MSCs osteogenesis in pediatric osteogenesis imperfecta.Biomed Pharmacother. 2024 Jun;175:116725. doi: 10.1016/j.biopha.2024.116725. Epub 2024 May 13. Biomed Pharmacother. 2024. PMID: 38744219
-
Induced pluripotent stem cells as a new getaway for bone tissue engineering: A systematic review.Cell Prolif. 2017 Apr;50(2):e12321. doi: 10.1111/cpr.12321. Epub 2016 Dec 1. Cell Prolif. 2017. PMID: 27905670 Free PMC article.
-
Mechanical stimulation-induced purinome priming fosters osteogenic differentiation and osteointegration of mesenchymal stem cells from the bone marrow of post-menopausal women.Stem Cell Res Ther. 2024 Jun 18;15(1):168. doi: 10.1186/s13287-024-03775-4. Stem Cell Res Ther. 2024. PMID: 38886849 Free PMC article.
-
TGF-β promotes the proliferation and osteogenic differentiation of dental pulp stem cells a systematic review and meta-analysis.Eur J Med Res. 2023 Jul 27;28(1):261. doi: 10.1186/s40001-023-01227-y. Eur J Med Res. 2023. PMID: 37501191 Free PMC article.
Cited by
-
When Bone Forms Where It Shouldn't: Heterotopic Ossification in Muscle Injury and Disease.Int J Mol Sci. 2025 Aug 4;26(15):7516. doi: 10.3390/ijms26157516. Int J Mol Sci. 2025. PMID: 40806642 Free PMC article. Review.
-
Application of Mesenchymal Stem Cells in Female Infertility Treatment: Protocols and Preliminary Results.Life (Basel). 2024 Sep 13;14(9):1161. doi: 10.3390/life14091161. Life (Basel). 2024. PMID: 39337944 Free PMC article. Review.
-
IRX3 controls a SUMOylation-dependent differentiation switch in adipocyte precursor cells.Nat Commun. 2025 Aug 6;16(1):7248. doi: 10.1038/s41467-025-62361-1. Nat Commun. 2025. PMID: 40769964 Free PMC article.
-
Oxidative Mechanism of Peyronie's Disease and Effectiveness of Pentoxifylline in the Therapeutic Management: A Narrative Review.Antioxidants (Basel). 2025 Feb 12;14(2):208. doi: 10.3390/antiox14020208. Antioxidants (Basel). 2025. PMID: 40002394 Free PMC article. Review.
-
Psoriasis: The Versatility of Mesenchymal Stem Cell and Exosome Therapies.Biomolecules. 2024 Oct 24;14(11):1351. doi: 10.3390/biom14111351. Biomolecules. 2024. PMID: 39595528 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 82370924/National Natural Science Foundation of China
- 82170929/National Natural Science Foundation of China
- 81970908/National Natural Science Foundation of China
- L222090/Beijing Natural Science Foundation-Haidian Original Innovation Joint Fund Project
- L222030/Beijing Natural Science Foundation-Haidian Original Innovation Joint Fund Project
LinkOut - more resources
Full Text Sources

