Neuromodulation of inhibitory control using phase-lagged transcranial alternating current stimulation
- PMID: 38816860
- PMCID: PMC11138099
- DOI: 10.1186/s12984-024-01385-y
Neuromodulation of inhibitory control using phase-lagged transcranial alternating current stimulation
Abstract
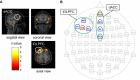
Background: Transcranial alternating current stimulation (tACS) is a prominent non-invasive brain stimulation method for modulating neural oscillations and enhancing human cognitive function. This study aimed to investigate the effects of individualized theta tACS delivered in-phase and out-of-phase between the dorsal anterior cingulate cortex (dACC) and left dorsolateral prefrontal cortex (lDLPFC) during inhibitory control performance.
Methods: The participants engaged in a Stroop task with phase-lagged theta tACS over individually optimized high-density electrode montages targeting the dACC and lDLPFC. We analyzed task performance, event-related potentials, and prestimulus electroencephalographic theta and alpha power.
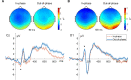
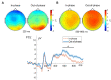
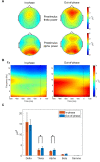
Results: We observed significantly reduced reaction times following out-of-phase tACS, accompanied by reduced frontocentral N1 and N2 amplitudes, enhanced parieto-occipital P1 amplitudes, and pronounced frontocentral late sustained potentials. Out-of-phase stimulation also resulted in significantly higher prestimulus frontocentral theta and alpha activity.
Conclusions: These findings suggest that out-of-phase theta tACS potently modulates top-down inhibitory control, supporting the feasibility of phase-lagged tACS to enhance inhibitory control performance.
Keywords: EEG; Inhibitory control; Non-invasive neuromodulation; Phase-lagging; Transcranial alternating current stimulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Out-of-phase transcranial alternating current stimulation modulates the neurodynamics of inhibitory control.Neuroimage. 2024 Apr 15;292:120612. doi: 10.1016/j.neuroimage.2024.120612. Epub 2024 Apr 20. Neuroimage. 2024. PMID: 38648868
-
Alpha-Oscillatory Current Application Impacts Prospective Remembering Through Strategic Monitoring.Psychophysiology. 2025 Mar;62(3):e70024. doi: 10.1111/psyp.70024. Psychophysiology. 2025. PMID: 40090877 Free PMC article.
-
Individual differences in resting alpha band power and changes in theta band power during sustained pain are correlated with the pain-relieving efficacy of alpha HD-tACS on SM1.Neuroimage. 2025 May 15;312:121237. doi: 10.1016/j.neuroimage.2025.121237. Epub 2025 Apr 23. Neuroimage. 2025. PMID: 40280214
-
Aligning Event-Related Potentials with Transcranial Alternating Current Stimulation for Modulation-a Review.Brain Topogr. 2024 Nov;37(6):933-946. doi: 10.1007/s10548-024-01055-1. Epub 2024 Apr 30. Brain Topogr. 2024. PMID: 38689065 Free PMC article. Review.
-
Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity.Neuroimage Clin. 2015 Mar 24;8:1-31. doi: 10.1016/j.nicl.2015.03.016. eCollection 2015. Neuroimage Clin. 2015. PMID: 26110109 Free PMC article. Review.
Cited by
-
Restorative Effects of Daytime Naps on Inhibitory Control: A Neuroimaging Study Following Sleep Deprivation.Nat Sci Sleep. 2025 Mar 18;17:475-487. doi: 10.2147/NSS.S499702. eCollection 2025. Nat Sci Sleep. 2025. PMID: 40124581 Free PMC article.
References
-
- Klirova M, Vorackova V, Horacek J, Mohr P, Jonas J, Dudysova DU, Kostylkova L, Fayette D, Krejcova L, Baumann S, et al. Modulating inhibitory control processes using Individualized High Definition Theta Transcranial Alternating current stimulation (HD theta-tACS) of the Anterior Cingulate and Medial Prefrontal Cortex. Front Syst Neurosci. 2021;15:611507. doi: 10.3389/fnsys.2021.611507. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

