The war between the immune system and the tumor - using immune biomarkers as tracers
- PMID: 38816871
- PMCID: PMC11137916
- DOI: 10.1186/s40364-024-00599-5
The war between the immune system and the tumor - using immune biomarkers as tracers
Abstract
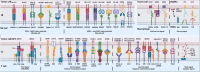
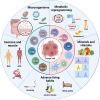
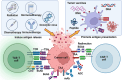
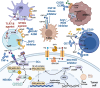
Nowadays, immunotherapy is one of the most promising anti-tumor therapeutic strategy. Specifically, immune-related targets can be used to predict the efficacy and side effects of immunotherapy and monitor the tumor immune response. In the past few decades, increasing numbers of novel immune biomarkers have been found to participate in certain links of the tumor immunity to contribute to the formation of immunosuppression and have entered clinical trials. Here, we systematically reviewed the oncogenesis and progression of cancer in the view of anti-tumor immunity, particularly in terms of tumor antigen expression (related to tumor immunogenicity) and tumor innate immunity to complement the cancer-immune cycle. From the perspective of integrated management of chronic cancer, we also appraised emerging factors affecting tumor immunity (including metabolic, microbial, and exercise-related markers). We finally summarized the clinical studies and applications based on immune biomarkers. Overall, immune biomarkers participate in promoting the development of more precise and individualized immunotherapy by predicting, monitoring, and regulating tumor immune response. Therefore, targeting immune biomarkers may lead to the development of innovative clinical applications.
Keywords: Cancer; Immune biomarker; Immunotherapy; Tumor immunity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
Grants and funding
- 81972484, 82272667/National Natural Science Foundation of China grants
- 81972484, 82272667/National Natural Science Foundation of China grants
- 81972484, 82272667/National Natural Science Foundation of China grants
- 81972484, 82272667/National Natural Science Foundation of China grants
- 81972484, 82272667/National Natural Science Foundation of China grants
- 81972484, 82272667/National Natural Science Foundation of China grants
- 81972484, 82272667/National Natural Science Foundation of China grants
- 81972484, 82272667/National Natural Science Foundation of China grants
- 81972484, 82272667/National Natural Science Foundation of China grants
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX102GSP201727/High-level Innovation Team of Nanjing Medical University Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- JX21817902/008/The Collaborative Innovation Center for Tumor Individualization Program
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
- Y-2019AZZD-00680/Beijing Xisike Clinical Oncology Research Foundation
LinkOut - more resources
Full Text Sources
Miscellaneous

