Photochemical dearomative skeletal modifications of heteroaromatics
- PMID: 38817197
- PMCID: PMC11181993
- DOI: 10.1039/d4cs00137k
Photochemical dearomative skeletal modifications of heteroaromatics
Abstract
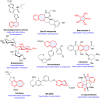
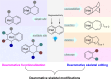
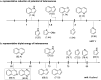
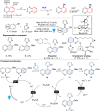
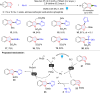
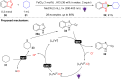
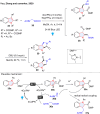
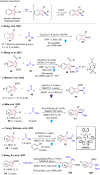
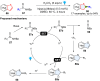
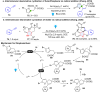
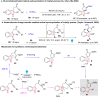
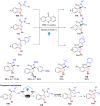
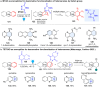
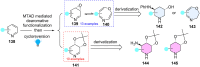
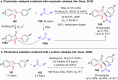
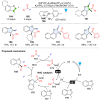
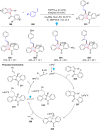
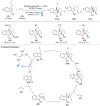
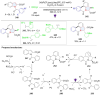
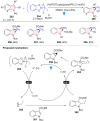
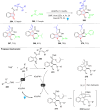
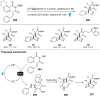
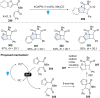
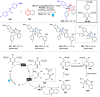
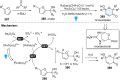
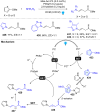
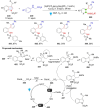
Dearomatization has emerged as a powerful tool for rapid construction of 3D molecular architectures from simple, abundant, and planar (hetero)arenes. The field has evolved beyond simple dearomatization driven by new synthetic technology development. With the renaissance of photocatalysis and expansion of the activation mode, the last few years have witnessed impressive developments in innovative photochemical dearomatization methodologies, enabling skeletal modifications of dearomatized structures. They offer truly efficient and useful tools for facile construction of highly complex structures, which are viable for natural product synthesis and drug discovery. In this review, we aim to provide a mechanistically insightful overview on these innovations based on the degree of skeletal alteration, categorized into dearomative functionalization and skeletal editing, and to highlight their synthetic utilities.
Conflict of interest statement
There are no conflicts to declare.
Figures























References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

