Acute and sub-chronic toxicity study of novel polyherbal formulation in non-alcoholic fatty liver using Wistar rats
- PMID: 38817372
- PMCID: PMC11137787
- DOI: 10.2144/fsoa-2023-0118
Acute and sub-chronic toxicity study of novel polyherbal formulation in non-alcoholic fatty liver using Wistar rats
Abstract
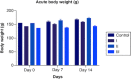
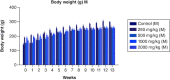
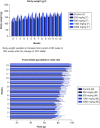
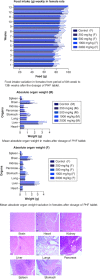
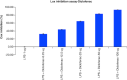
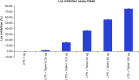
Aim: This study assessed the acute and sub-chronic toxicity of a novel polyherbal formulation tablet in Wistar rats Materials & methods: Acute toxicity and sub-chronic toxicity was assessed following OECD (Organisation for the Economic Co-operation and Development) guidelines based on 423 and 408. Results & conclusion: No mortality and toxicity showed in rats during acute toxicity. The LD50 of the extract was at 2000 mg/kg. In sub-chronic study, both sex rats were orally administered at 250, 500,1000 and 2000 mg/kg for 90 days and revealed no significant difference (p < 0.05) in hematological and other parameters compared with the control. Histopathology study did not reveal morphological alteration. The No observed adverse effect level of the tablet was observed until 2000 mg/kg.
Keywords: NOAEL; PHF tablet; acute toxicity; non-alcoholic fatty liver; sub-chronic toxicity.
Plain language summary
Non-alcoholic fatty liver disease (NAFLD) affects around 25% of individuals globally and has become the most common long-term liver problem. The reasons why people get this disease can be different for each person. By studying natural substances, scientists have discovered that some compounds help treat the disease some of these substances can also be harmful. By studying natural substances, scientists have discovered that some compounds help treat the disease some of these substances can also be harmful. People are also trying out traditional medicines more and more, and we need to make sure they're safe. To determine whether a medication is secure, we conducted experiments in accordance with the OECD guidelines. One test examines whether a high dose of the drug is lethal. The goal is to determine the optimal dose, which is neither too low nor too excessive. Another test investigated what happens if these rats take the medicine every day for a long time. Variables such as blood tests and tissue samples are collected to make sure the medicine does not make the rats sick. In this case, we tested a medicine called a ‘PHF tablet’ for 90 days, and it didn't make the animals sick. They found that you can take a relatively high dose without any adverse effects.
© 2024 The Authors.
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures




References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1), 73–84 (2016). - PubMed
-
- Younossi Z, Anstee QM, Marietti Met al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology. 15(1), 11–20 (2018). - PubMed
-
- Angulo P, Kleiner DE, Dam-Larsen Set al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149(2), 389–397.e310 (2015). - PMC - PubMed
-
•• This article is of considerable interest, as it denotes about the histologic features and the outcome of the fibrosis in NAFLD patient.
-
- Fan J-G, Kim S-U, Wong VW-S. New trends on obesity and NAFLD in Asia. J. Hepatol. 67(4), 862–873 (2017). - PubMed
-
• This article is of interest, as it denotes about the trends of obesity, NAFLD and NASH in Asia. The Asian patients are however, under presented in any of the clinical trials as this disease remain very uncommon in Asian population.
-
- Lambert JE, Ramos–Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146(3), 726–735 (2014). - PMC - PubMed
-
•• This article is of considerable interest, as it denotes about the NAFLD patients who had higher nocturnal plasma levels of FFAs and did not suppress the contribution from de novo lipogenesis on fasting.
LinkOut - more resources
Full Text Sources
Research Materials
