Demonstration of tritium adsorption on graphene
- PMID: 38817427
- PMCID: PMC11134268
- DOI: 10.1039/d3na00904a
Demonstration of tritium adsorption on graphene
Abstract
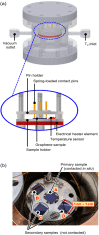
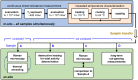
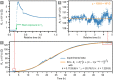
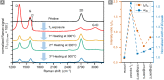
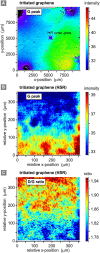
In this work, we report on studies of graphene exposed to tritium gas in a controlled environment. The single layer graphene on a SiO2/Si substrate was exposed to 400 mbar of T2, for a total time of ∼55 h. The resistivity of the graphene sample was measured in situ during tritium exposure using the van der Pauw method. We found that the sheet resistance increases by three orders of magnitude during the exposure, suggesting significant chemisorption of tritium. After exposure, the samples were characterised ex situ via spatio-chemical mapping with a confocal Raman microscope, to study the effect of tritium on the graphene structure (tritiation yielding T-graphene), as well as the homogeneity of modifications across the whole area of the graphene film. The Raman spectra after tritium exposure were comparable to previously observed results in hydrogen-loading experiments, carried out by other groups. By thermal annealing we also could demonstrate, using Raman spectral analysis, that the structural changes were largely reversible. Considering all observations, we conclude that the graphene film was at least partially tritiated during the tritium exposure, and that the graphene film by and large withstands the bombardment by electrons from the β-decay of tritium, as well as by energetic primary and secondary ions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
LinkOut - more resources
Full Text Sources

