Structural elucidation of HIV-1 G-quadruplexes in a cellular environment and their ligand binding using responsive 19F-labeled nucleoside probes
- PMID: 38817587
- PMCID: PMC11134374
- DOI: 10.1039/d4sc01755b
Structural elucidation of HIV-1 G-quadruplexes in a cellular environment and their ligand binding using responsive 19F-labeled nucleoside probes
Abstract
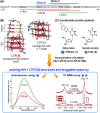
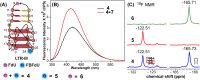
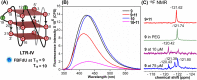
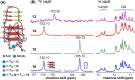
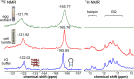
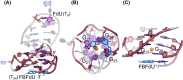
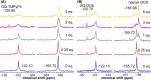
Understanding the structure and recognition of highly conserved regulatory segments of the integrated viral DNA genome that forms unique topologies can greatly aid in devising novel therapeutic strategies to counter chronic infections. In this study, we configured a probe system using highly environment-sensitive nucleoside analogs, 5-fluoro-2'-deoxyuridine (FdU) and 5-fluorobenzofuran-2'-deoxyuridine (FBFdU), to investigate the structural polymorphism of HIV-1 long terminal repeat (LTR) G-quadruplexes (GQs) by fluorescence and 19F NMR. FdU and FBFdU, serving as hairpin and GQ sensors, produced distinct spectral signatures for different GQ topologies adopted by LTR G-rich oligonucleotides. Importantly, systematic 19F NMR analysis in Xenopus laevis oocytes gave unprecedented information on the structure adopted by the LTR G-rich region in the cellular environment. The results indicate that it forms a unique GQ-hairpin hybrid architecture, a potent hotspot for selective targeting. Furthermore, structural models generated using MD simulations provided insights on how the probe system senses different GQs. Using the responsiveness of the probes and Taq DNA polymerase stop assay, we monitored GQ- and hairpin-specific ligand interactions and their synergistic inhibitory effect on the replication process. Our findings suggest that targeting GQ and hairpin motifs simultaneously using bimodal ligands could be a new strategy to selectively block the viral replication.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Gupta R. K. Gregson J. Parkin N. Haile-Selassie H. Tanuri A. Andrade F. L. Kaleebu P. Watera C. Aghokeng A. Mutenda N. Dzangare J. Hone S. Hang Z. Z. Garcia J. Garcia Z. Marchorro P. Beteta E. Giron A. Hamers R. Inzaule S. Frenkel L. M. Chung M. H. de Oliveira T. Pillay D. Naidoo K. Kharsany A. Kugathasan R. Cutino T. Hunt G. Rios S. A. Doherty M. Jordan M. R. Bertagnolio S. Lancet Infect. Dis. 2018;18:346–355. doi: 10.1016/S1473-3099(17)30702-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

