Formulation, development and evaluation of hyaluronic acid-conjugated liposomal nanoparticles loaded with regorafenib and curcumin and their in vitro evaluation on colorectal cancer cell lines
- PMID: 38817822
- PMCID: PMC11135027
- DOI: 10.1016/j.jsps.2024.102099
Formulation, development and evaluation of hyaluronic acid-conjugated liposomal nanoparticles loaded with regorafenib and curcumin and their in vitro evaluation on colorectal cancer cell lines
Abstract
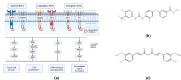
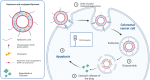
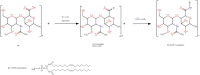
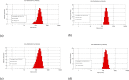
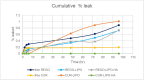
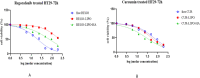
Colorectal cancer is one of the major causes of global cancer, with chemotherapy and radiation therapy being effective but limited due to low specificity. Regorafenib, a multikinase inhibitor, provides hope to patients with metastatic colorectal cancer and was approved by the FDA in 2012. However, due to resistance issues and adverse events, its efficacy is compromised, necessitating further refinement. Meanwhile, curcumin, a compound of turmeric, exhibits anticancer effects through antioxidant and anti-inflammatory actions, induction of the apoptosis, arrest of cell cycle, inhibition of angiogenesis, and modulation of signaling pathways. Unfortunately, its clinical utility is limited by its poor bioavailability, pointing towards innovative drug delivery strategies for enhanced efficacy in colorectal cancer treatment. Hyaluronic acid (HA)-decorated liposomes (LIPO) have been developed to target colorectal cells through an overexpressed CD44 receptor, increasing antitumor and antimetastasis efficacy. This study investigates the possibility of loading curcumin (CUR) or regorafenib (REGO) into a liposomal formulation for passive and HA-actively targeted treatment, evaluating its critical quality attributes (CQA) (size, zeta potential, polydispersity index) and cytotoxic activity in the HT29 colorectal cancer cell line. The average particle size of the plain liposomes and those decorated with HA was 144.00 ± 0.78 nm and 140.77 ± 1.64 nm, respectively. In contrast, curcumin-loaded plain liposomes and HA-decorated liposomes had 140 ± 2.46 nm and 164.53 ± 15.13 nm, respectively. The prepared liposomes had a spherical shape with a narrow size distribution and an acceptable zeta potential of less than -30 mV. The encapsulation efficiency was 99.2 % ± 0.3 and 99.9 ± 0.2 % for HA-decorated and bare regorafenib loaded. The % EE was 98.9 ± 0.2 % and 97.5 ± 0.2 % for bare liposomal nanoparticles loaded with curcumin and coated with curcumin. The IC50 of free REGO, CUR, REGO-LIPO, CUR-LIPO, REGO-LIPO-HA and CUR-LIPO-HA were 20.17 ± 0.78, 64.4 ± 0.33, 224.8 ± 0.06, 49.66 ± 0.22, 73.66 ± 0.6, and 27.86 ± 0.49 µM, respectively. The MTT assay in HT29 cells showed significant cytotoxic activity of the HA-decorated liposomal formulation compared to the base uncoated formulation, indicating that hyaluronic acid-targeted liposomes loaded with regorafenib or curcumin could be a promising targeted formulation against colorectal cancer cells.
Keywords: CD44; Colorectal cancer; Curcumin; Hyaluronic acid; Liposome; Regorafenib; Targeted drug delivery.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Barbălată C.I., Porfire A.S., Sesarman A., Rauca V.-F., Banciu M., Muntean D., Știufiuc R., Moldovan A., Moldovan C., Tomuță I. A screening study for the development of simvastatin-doxorubicin liposomes, a Co-formulation with future perspectives in colon cancer therapy. Pharmaceutics. 2021;13(10):1526. doi: 10.3390/pharmaceutics13101526. - DOI - PMC - PubMed
-
- Bardania H., Jafari F., Baneshi M., Mahmoudi R., Ardakani M.T., Safari F., Barmak M.J. Folic acid-functionalized albumin/graphene oxide nanocomposite to simultaneously deliver curcumin and 5-fluorouracil into human colorectal cancer cells: an in vitro study. Biomed Res. Int. 2023;2023:e8334102. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

