Heterogeneous Response of Tumor Cell Lines to Inhibition of Aspartate β-hydroxylase
- PMID: 38817852
- PMCID: PMC11134442
- DOI: 10.7150/jca.94452
Heterogeneous Response of Tumor Cell Lines to Inhibition of Aspartate β-hydroxylase
Abstract
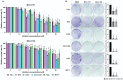
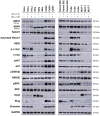
Background: Cancer development involves alterations in key cellular pathways, with aspartate β-hydroxylase (ASPH) emerging as an important player in tumorigenesis. ASPH is upregulated in various cancer types, where it promotes cancer progression mainly by regulating the Notch1 and SRC pathways. Methods: This study explored the responses of various human cervical, pharyngeal, and breast tumor cell lines to second- and third-generation ASPH inhibitors (MO-I-1151 and MO-I-1182) using proliferation, migration, and invasion assays; western blotting; and cell cycle analysis. Results: ASPH inhibition significantly reduced cell proliferation, migration, and invasion and disrupted both the canonical and noncanonical Notch1 pathways. The noncanonical pathway was particularly mediated by AKT signaling. Cell cycle analysis revealed a marked reduction in cyclin D1 expression, further confirming the inhibitory effect of ASPH inhibitors on cell proliferation. Additional analysis revealed G0/G1 arrest and restricted progression into S phase, highlighting the regulatory impact of ASPH inhibitors on the cell cycle. Furthermore, ASPH inhibition induced distinctive alterations in nuclear morphology. The high heterogeneity in the responses of individual tumor cell lines to ASPH inhibitors, both quantitatively and qualitatively, underscores the complex network of mechanisms that are regulated by ASPH and influence the efficacy of ASPH inhibition. The effects of ASPH inhibitors on Notch1 pathway activity, cyclin D1 expression, and nuclear morphology contribute to the understanding of the multifaceted effects of these inhibitors on cancer cell behavior. Conclusion: This study not only suggests that ASPH inhibitors are effective against tumor cell progression, in part through the induction of cell cycle arrest, but also highlights the diverse and heterogeneous effects of these inhibitors on the behavior of tumor cells of different origins.
Keywords: AKT signaling; ASPH inhibitors; Notch pathway; cell cycle; heterogeneity; tumorigenesis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Greve JM, Pinkham AM, Cowan JA. Human aspartyl (asparaginyl) hydroxylase. A multifaceted enzyme with broad intra- and extra-cellular activity. Metallomics. 2021;13:mfab044. - PubMed
-
- Dinchuk JE, Henderson NL, Burn TC. et al. Aspartyl β-hydroxylase (Asph) and an evolutionarily conserved isoform of Asph missing the catalytic domain share exons with junctin. J Biol Chem. 2000;275:39543–54. - PubMed
-
- Engel J. EGF-like domains in extracellular matrix proteins: Localized signals for growth and differentiation? FEBS Lett. 1989;251:1–7. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

