Neutrophil Extracellular Traps in Breast Cancer: Roles in Metastasis and Beyond
- PMID: 38817858
- PMCID: PMC11134451
- DOI: 10.7150/jca.94669
Neutrophil Extracellular Traps in Breast Cancer: Roles in Metastasis and Beyond
Abstract
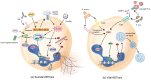
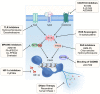
Despite advances in the treatment of breast cancer, the disease continues to exhibit high global morbidity and mortality. The importance of neutrophils in cancer development has been increasingly recognized. Neutrophil extracellular traps (NETs) are web-like structures released into the extracellular space by activated neutrophils, serving as a potential antimicrobial mechanism for capturing and eliminating microorganisms. The roles played by NETs in cancer development have been a subject of intense research in the last decade. In breast cancer, current evidence suggests that NETs are involved in various stages of cancer development, particularly during metastasis. In this review, we try to provide an updated overview of the roles played by NETs in breast cancer metastasis. These include: 1) facilitating systemic dissemination of cancer cells; 2) promoting cancer-associated inflammation; 3) facilitating cancer-associated thrombosis; 4) facilitating pre-metastatic niche formation; and 5) awakening dormant cancer cells. The translational implications of NETs in breast cancer treatment are also discussed. Understanding the relationship between NETs and breast cancer metastasis is expected to provide important insights for developing new therapeutic strategies for breast cancer patients.
Keywords: Breast cancer; Neutrophil extracellular trap (NET); Therapeutic target; Tumor immunity; Tumor microenvironment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Advances in Targeting Neutrophil Extracellular Traps as a Promising Approach for Breast Cancer Treatment.Comb Chem High Throughput Screen. 2025 Feb 26. doi: 10.2174/0113862073376243250130060239. Online ahead of print. Comb Chem High Throughput Screen. 2025. PMID: 40012387
-
Neutrophil Extracellular Traps (NETs) in Cancer Metastasis.Cancers (Basel). 2021 Dec 6;13(23):6131. doi: 10.3390/cancers13236131. Cancers (Basel). 2021. PMID: 34885240 Free PMC article. Review.
-
The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis.Front Immunol. 2020 Sep 16;11:1749. doi: 10.3389/fimmu.2020.01749. eCollection 2020. Front Immunol. 2020. PMID: 33042107 Free PMC article. Review.
-
Neutrophil extracellular traps in cancer.MedComm (2020). 2024 Jul 15;5(8):e647. doi: 10.1002/mco2.647. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39015554 Free PMC article. Review.
-
Neutrophil Extracellular Traps (NETs) in Cancer Invasion, Evasion and Metastasis.Cancers (Basel). 2021 Sep 6;13(17):4495. doi: 10.3390/cancers13174495. Cancers (Basel). 2021. PMID: 34503307 Free PMC article. Review.
Cited by
-
Neutrophil extracellular traps in diseases of the female reproductive organs.Front Immunol. 2025 May 5;16:1589329. doi: 10.3389/fimmu.2025.1589329. eCollection 2025. Front Immunol. 2025. PMID: 40391215 Free PMC article. Review.
-
Cryoablation-induced neutrophil Ca2+ elevation and NET formation exacerbate immune escape in colorectal cancer liver metastasis.J Exp Clin Cancer Res. 2024 Dec 9;43(1):319. doi: 10.1186/s13046-024-03244-z. J Exp Clin Cancer Res. 2024. PMID: 39648199 Free PMC article.
-
The Dual Roles of Circular RNAs in Breast Cancer Distant Metastasis and Their Clinical Applications.J Cancer. 2025 Jul 11;16(10):3270-3282. doi: 10.7150/jca.111680. eCollection 2025. J Cancer. 2025. PMID: 40740236 Free PMC article. Review.
-
Unraveling the complex role of tumor-associated neutrophils within solid tumors.Cancer Immunol Immunother. 2025 May 19;74(7):210. doi: 10.1007/s00262-025-04049-5. Cancer Immunol Immunother. 2025. PMID: 40387965 Free PMC article. Review.
-
Antioxidants as Modulators of NETosis: Mechanisms, Evidence, and Therapeutic Potential.Int J Mol Sci. 2025 May 30;26(11):5272. doi: 10.3390/ijms26115272. Int J Mol Sci. 2025. PMID: 40508099 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Rosales C. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol. 2020;108:377–96. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

