N-to- S Acyl Transfer as an Enabling Strategy in Asymmetric and Chemoenzymatic Synthesis
- PMID: 38818054
- PMCID: PMC11134368
- DOI: 10.1021/jacsau.4c00257
N-to- S Acyl Transfer as an Enabling Strategy in Asymmetric and Chemoenzymatic Synthesis
Abstract
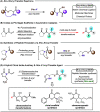
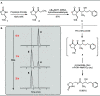
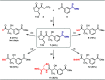
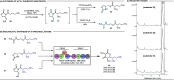
The observation of thioester-mediated acyl transfer processes in nature has inspired the development of novel protein synthesis and functionalization methodologies. The chemoselective transfer of an acyl group from S-to-N is the basis of several powerful ligation strategies. In this work, we sought to apply the reverse process, the transfer of an acyl group from N-to-S, as a method to convert stable chiral amides into more reactive thioesters. To this end, we developed a novel cysteine-derived oxazolidinone that serves as both a chiral imide auxiliary and an acyl transfer agent. This auxiliary combines the desirable features of rigid chiral imides as templates for asymmetric transformations with the synthetic applicability of thioesters. We demonstrate that the auxiliary can be applied in a range of highly selective asymmetric transformations. Subsequent intramolecular N-to-S acyl transfer of the chiral product and in situ trapping of the resulting thioester provides access to diverse carboxylic acid derivatives under mild conditions. The oxazolidinone thioester products can also be isolated and used in Pd-mediated transformations to furnish highly valuable chiral scaffolds, such as noncanonical amino acids, cyclic ketones, tetrahydropyrones, and dihydroquinolinones. Finally, we demonstrate that the oxazolidinone thioesters can also serve as a surrogate for SNAC-thioesters, enabling their seamless use as non-native substrates in biocatalytic transformations.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
-
For reviews, see:
- Hirschbeck V.; Gehrtz P. H.; Fleischer I. Metal-Catalyzed Synthesis and Use of Thioesters: Recent Developments. Chem. – Eur. J. 2018, 24 (28), 7092–7107. 10.1002/chem.201705025. - DOI - PubMed
- Cheng H.; Chen H.; Liu Y.; Zhou Q. The Liebeskind–Srogl Cross-Coupling Reaction and Its Synthetic Applications. Asian J. Org. Chem. 2018, 7 (3), 490–508. 10.1002/ajoc.201700651. - DOI
- Sikandar S.; Zahoor A. F.; Naheed S.; Parveen B.; Ali K. G.; Akhtar R. Fukuyama Reduction, Fukuyama Coupling and Fukuyama–Mitsunobu Alkylation: Recent Developments and Synthetic Applications. Mol. Divers. 2022, 26, 589–628. 10.1007/s11030-021-10194-7. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
