CDK2 and CDK4: Cell Cycle Functions Evolve Distinct, Catalysis-Competent Conformations, Offering Drug Targets
- PMID: 38818077
- PMCID: PMC11134382
- DOI: 10.1021/jacsau.4c00138
CDK2 and CDK4: Cell Cycle Functions Evolve Distinct, Catalysis-Competent Conformations, Offering Drug Targets
Abstract
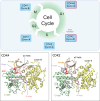
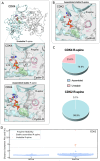
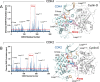
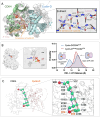
Cyclin-dependent kinases (CDKs), particularly CDK4 and CDK2, are crucial for cell cycle progression from the Gap 1 (G1) to the Synthesis (S) phase by phosphorylating targets such as the Retinoblastoma Protein (Rb). CDK4, paired with cyclin-D, operates in the long G1 phase, while CDK2 with cyclin-E, manages the brief G1-to-S transition, enabling DNA replication. Aberrant CDK signaling leads to uncontrolled cell proliferation, which is a hallmark of cancer. Exactly how they accomplish their catalytic phosphorylation actions with distinct efficiencies poses the fundamental, albeit overlooked question. Here we combined available experimental data and modeling of the active complexes to establish their conformational functional landscapes to explain how the two cyclin/CDK complexes differentially populate their catalytically competent states for cell cycle progression. Our premise is that CDK catalytic efficiencies could be more important for cell cycle progression than the cyclin-CDK biochemical binding specificity and that efficiency is likely the prime determinant of cell cycle progression. We observe that CDK4 is more dynamic than CDK2 in the ATP binding site, the regulatory spine, and the interaction with its cyclin partner. The N-terminus of cyclin-D acts as an allosteric regulator of the activation loop and the ATP-binding site in CDK4. Integrated with a suite of experimental data, we suggest that the CDK4 complex is less capable of remaining in the active catalytically competent conformation, and may have a lower catalytic efficiency than CDK2, befitting their cell cycle time scales, and point to critical residues and motifs that drive their differences. Our mechanistic landscape may apply broadly to kinases, and we propose two drug design strategies: (i) allosteric Inhibition by conformational stabilization for targeting allosteric CDK4 regulation by cyclin-D, and (ii) dynamic entropy-optimized targeting which leverages the dynamic, entropic aspects of CDK4 to optimize drug binding efficacy.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Morgan D. O.The Cell Cycle: Principles of Control; New Science Press, 2007.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
