Cancer-Stem-Cell Phenotype-Guided Discovery of a Microbiota-Inspired Synthetic Compound Targeting NPM1 for Leukemia
- PMID: 38818079
- PMCID: PMC11134387
- DOI: 10.1021/jacsau.3c00682
Cancer-Stem-Cell Phenotype-Guided Discovery of a Microbiota-Inspired Synthetic Compound Targeting NPM1 for Leukemia
Abstract
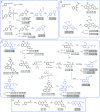
The human microbiota plays an important role in human health and disease, through the secretion of metabolites that regulate key biological functions. We propose that microbiota metabolites represent an unexplored chemical space of small drug-like molecules in the search of new hits for drug discovery. Here, we describe the generation of a set of complex chemotypes inspired on selected microbiota metabolites, which have been synthesized using asymmetric organocatalytic reactions. Following a primary screening in CSC models, we identified the novel compound UCM-13369 (4b) whose cytotoxicity was mediated by NPM1. This protein is one of the most frequent mutations of AML, and NPM1-mutated AML is recognized by the WHO as a distinct hematopoietic malignancy. UCM-13369 inhibits NPM1 expression, downregulates the pathway associated with mutant NPM1 C+, and specifically recognizes the C-end DNA-binding domain of NPM1 C+, avoiding the nucleus-cytoplasm translocation involved in the AML tumorological process. The new NPM1 inhibitor triggers apoptosis in AML cell lines and primary cells from AML patients and reduces tumor infiltration in a mouse model of AML with NPM1 C+ mutation. The disclosed phenotype-guided discovery of UCM-13369, a novel small molecule inspired on microbiota metabolites, confirms that CSC death induced by NPM1 inhibition represents a promising therapeutic opportunity for NPM1-mutated AML, a high-mortality disease.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
LinkOut - more resources
Full Text Sources
