Charge Regulation Triggers Condensation of Short Oligopeptides to Polyelectrolytes
- PMID: 38818083
- PMCID: PMC11134362
- DOI: 10.1021/jacsau.3c00668
Charge Regulation Triggers Condensation of Short Oligopeptides to Polyelectrolytes
Abstract
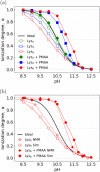
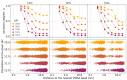
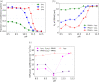
Electrostatic interactions between charged macromolecules are ubiquitous in biological systems, and they are important also in materials design. Attraction between oppositely charged molecules is often interpreted as if the molecules had a fixed charge, which is not affected by their interaction. Less commonly, charge regulation is invoked to interpret such interactions, i.e., a change of the charge state in response to a change of the local environment. Although some theoretical and simulation studies suggest that charge regulation plays an important role in intermolecular interactions, experimental evidence supporting such a view is very scarce. In the current study, we used a model system, composed of a long polyanion interacting with cationic oligolysines, containing up to 8 lysine residues. We showed using both simulations and experiments that while these lysines are only weakly charged in the absence of the polyanion, they charge up and condense on the polycations if the pH is close to the pKa of the lysine side chains. We show that the lysines coexist in two distinct populations within the same solution: (1) practically nonionized and free in solution; (2) highly ionized and condensed on the polyanion. Using this model system, we demonstrate under what conditions charge regulation plays a significant role in the interactions of oppositely charged macromolecules and generalize our findings beyond the specific system used here.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
