Portable spectroscopy, digital imaging colorimetry and multivariate statistical tools in contaminant identification: A case study of mint (Mentha) and basil (Ocimum basilicum)
- PMID: 38818158
- PMCID: PMC11137394
- DOI: 10.1016/j.heliyon.2024.e30924
Portable spectroscopy, digital imaging colorimetry and multivariate statistical tools in contaminant identification: A case study of mint (Mentha) and basil (Ocimum basilicum)
Abstract
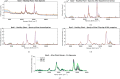
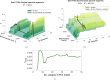
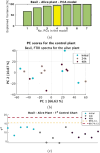
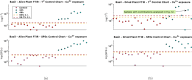
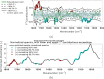
The advent of portable Fourier-Transform Infrared (FTIR) and Raman spectrometers has revolutionized analysis capabilities, presenting the possibility of on-site contaminant identification without the need for specialized laboratory settings. Compared to laboratory instrumentation, portable spectroscopy is more prone to noise, and appropriate spectral processing procedures need to be established. This paper introduces a comprehensive methodology that integrates acquisition techniques, spectral analysis, and mathematical tools necessary for utilizing handheld spectrometers to diagnose plant contamination. It focuses on determining the efficacy of handheld FTIR, Raman spectroscopy, and digital imaging for detecting contaminants in two food plants, Basil (Ocimum basilicum) and Mint (Mentha). The study examines the impact of three pollutants: iron (II) sulphate (), zinc (II) sulphate (), and copper (II) sulphate (), on these plants, but also the necessary amount of measurements to spot the pollutants' effects. Measurements were conducted at the start, after 24 hours, and after 48 hours of exposure, on both fresh and dried plant leaves, as well as in solution. Spectral effects of each of the pollutants were identified with the use of multivariate statistical process control techniques. With the help of the developed methodologies, researchers can identify in-situ contaminant effects, exposure times and run diagnostics directly on the leaf both in alive and dried plants.
Keywords: Colorimetry; Fourier-transform infrared (FTIR) spectroscopy; Handheld instrumentation; Plant contamination; Raman spectroscopy.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





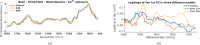

References
-
- Ivanova D.G., Singh B.R. Nondestructive FTIR monitoring of leaf senescence and elicitin-induced changes in plant leaves. Biopolymers, Orig. Res. Biomol. 2003;72:79–85. - PubMed
-
- Moskal P., Wesełucha-Birczyńska A., Łabanowska M., Filek M. Adaxial and abaxial pattern of urtica dioica leaves analyzed by 2dcos atr-ftir as a function of their growth time and impact of environmental pollution. Vib. Spectrosc. 2019;104
-
- Suresh S., Karthikeyan S., Jayamoorthy K. Ftir and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J. Sci. Adv. Mater. Dev. 2016;1:343–350.
-
- Westworth S., Ashwath N., Cozzolino D. Application of ftir-atr spectroscopy to detect salinity response in beauty leaf tree (calophyllum inophyllum l) Energy Proc. 2019;160:761–768.
LinkOut - more resources
Full Text Sources

