Variation in resource competition traits among Microcystis strains is affected by their microbiomes
- PMID: 38818269
- PMCID: PMC10989160
- DOI: 10.1002/mlf2.12094
Variation in resource competition traits among Microcystis strains is affected by their microbiomes
Abstract
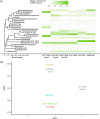
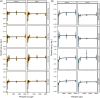
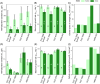
Freshwater harmful algal blooms are often dominated by Microcystis, a phylogenetically cohesive group of cyanobacteria marked by extensive genetic and physiological diversity. We have previously shown that this genetic diversity and the presence of a microbiome of heterotrophic bacteria influences competitive interactions with eukaryotic phytoplankton. In this study, we sought to explain these observations by characterizing Monod equation parameters for resource usage (maximum growth rate μ max, half-saturation value for growth K s, and quota) as a function of N and P levels for four strains (NIES-843, PCC 9701, PCC 7806 [WT], and PCC 7806 ΔmcyB) in presence and absence of a microbiome derived from Microcystis isolated from Lake Erie. Results indicated limited differences in maximum growth rates but more pronounced differences in half-saturation values among Microcystis strains. The largest impact of the microbiome was reducing the minimal nitrogen concentration sustaining growth and reducing half saturation values, with variable results depending on the Microcystis strain. Microcystis strains also differed from each other in their N and P quotas and the extent to which microbiome presence affected them. Our data highlight the importance of the microbiome in altering Microcystis-intrinsic traits, strain competitive hierarchies, and thus bloom dynamics. As quota, μ max, and K s are commonly used in models for harmful algal blooms, our data suggest that model improvement may be possible by incorporating genotype dependencies of resource-use parameters.
Keywords: cultivation‐dependent; fitness; harmful algal blooms; host–microbe interactions; phytoplankton.
© 2023 The Authors. mLife published by John Wiley & Sons Australia, Ltd on behalf of Institute of Microbiology, Chinese Academy of Sciences.
Conflict of interest statement
The authors declare no conflict of interests.
Figures


References
-
- Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JMH, Visser PM. Cyanobacterial blooms. Nat Rev Microbiol. 2018;16:471–483. - PubMed
-
- Gobler CJ. Climate change and harmful algal blooms: insights and perspective. Harmful Algae. 2020;91:101731. - PubMed
-
- Vanderploeg HA, Liebig JR, Carmichael WW, Agy MA, Johengen TH, Fahnenstiel GL, et al. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Can J Fish Aquat Sci. 2001;58:1208–1221.
LinkOut - more resources
Full Text Sources
Research Materials
