"Life is short, and art is long": RNA degradation in cyanobacteria and model bacteria
- PMID: 38818322
- PMCID: PMC10989914
- DOI: 10.1002/mlf2.12015
"Life is short, and art is long": RNA degradation in cyanobacteria and model bacteria
Abstract
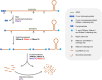
RNA turnover plays critical roles in the regulation of gene expression and allows cells to respond rapidly to environmental changes. In bacteria, the mechanisms of RNA turnover have been extensively studied in the models Escherichia coli and Bacillus subtilis, but not much is known in other bacteria. Cyanobacteria are a diverse group of photosynthetic organisms that have great potential for the sustainable production of valuable products using CO2 and solar energy. A better understanding of the regulation of RNA decay is important for both basic and applied studies of cyanobacteria. Genomic analysis shows that cyanobacteria have more than 10 ribonucleases and related proteins in common with E. coli and B. subtilis, and only a limited number of them have been experimentally investigated. In this review, we summarize the current knowledge about these RNA-turnover-related proteins in cyanobacteria. Although many of them are biochemically similar to their counterparts in E. coli and B. subtilis, they appear to have distinct cellular functions, suggesting a different mechanism of RNA turnover regulation in cyanobacteria. The identification of new players involved in the regulation of RNA turnover and the elucidation of their biological functions are among the future challenges in this field.
Keywords: RNA maturation; RNA metabolism; RNA turnover; cyanobacteria; ribonucleases.
© 2022 The Authors. mLife published by John Wiley & Sons Australia, Ltd. on behalf of Institute of Microbiology, Chinese Academy of Sciences.
Figures



Similar articles
-
Genetic and genomic analysis of RNases in model cyanobacteria.Photosynth Res. 2015 Oct;126(1):171-83. doi: 10.1007/s11120-015-0076-2. Epub 2015 Jan 17. Photosynth Res. 2015. PMID: 25595545 Free PMC article.
-
Dynamic Membrane Localization of RNase Y in Bacillus subtilis.mBio. 2020 Feb 18;11(1):e03337-19. doi: 10.1128/mBio.03337-19. mBio. 2020. PMID: 32071272 Free PMC article.
-
mRNA degradation and maturation in prokaryotes: the global players.Biomol Concepts. 2011 Dec 1;2(6):491-506. doi: 10.1515/BMC.2011.042. Biomol Concepts. 2011. PMID: 25962050
-
Function, mechanism and regulation of bacterial ribonucleases.FEMS Microbiol Rev. 1999 Jun;23(3):371-90. doi: 10.1111/j.1574-6976.1999.tb00405.x. FEMS Microbiol Rev. 1999. PMID: 10371039 Review.
-
Bacterial ribonucleases and their roles in RNA metabolism.Crit Rev Biochem Mol Biol. 2019 Jun;54(3):242-300. doi: 10.1080/10409238.2019.1651816. Crit Rev Biochem Mol Biol. 2019. PMID: 31464530 Free PMC article. Review.
Cited by
-
EbsA is essential for both motility and biofilm formation in the filamentous cyanobacterium Nostoc punctiforme.Microbiology (Reading). 2024 Sep;170(9):001498. doi: 10.1099/mic.0.001498. Microbiology (Reading). 2024. PMID: 39287971 Free PMC article.
-
Regulation of RNase E during the UV stress response in the cyanobacterium Synechocystis sp. PCC 6803.mLife. 2023 Feb 15;2(1):43-57. doi: 10.1002/mlf2.12056. eCollection 2023 Mar. mLife. 2023. PMID: 38818332 Free PMC article.
-
The role of the 5' sensing function of ribonuclease E in cyanobacteria.RNA Biol. 2024 Jan;21(1):1-18. doi: 10.1080/15476286.2024.2328438. Epub 2024 Mar 12. RNA Biol. 2024. PMID: 38469716 Free PMC article.
-
Regulation of pSYSA defense plasmid copy number in Synechocystis through RNase E and a highly transcribed asRNA.Front Microbiol. 2023 Feb 16;14:1112307. doi: 10.3389/fmicb.2023.1112307. eCollection 2023. Front Microbiol. 2023. PMID: 36876071 Free PMC article.
-
Improved RNA stability estimation indicates that transcriptional interference is frequent in diverse bacteria.Commun Biol. 2023 Jul 15;6(1):732. doi: 10.1038/s42003-023-05097-2. Commun Biol. 2023. PMID: 37454177 Free PMC article.
References
-
- Mohanty BK, Kushner SR. Regulation of mRNA Decay in Bacteria. Annu Rev Microbiol. 2016;70:25–44. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
