Regulation of RNase E during the UV stress response in the cyanobacterium Synechocystis sp. PCC 6803
- PMID: 38818332
- PMCID: PMC10989929
- DOI: 10.1002/mlf2.12056
Regulation of RNase E during the UV stress response in the cyanobacterium Synechocystis sp. PCC 6803
Abstract
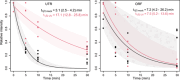
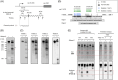
Endoribonucleases govern the maturation and degradation of RNA and are indispensable in the posttranscriptional regulation of gene expression. A key endoribonuclease in Gram-negative bacteria is RNase E. To ensure an appropriate supply of RNase E, some bacteria, such as Escherichia coli, feedback-regulate RNase E expression via the rne 5'-untranslated region (5' UTR) in cis. However, the mechanisms involved in the control of RNase E in other bacteria largely remain unknown. Cyanobacteria rely on solar light as an energy source for photosynthesis, despite the inherent ultraviolet (UV) irradiation. In this study, we first investigated globally the changes in gene expression in the cyanobacterium Synechocystis sp. PCC 6803 after a brief exposure to UV. Among the 407 responding genes 2 h after UV exposure was a prominent upregulation of rne mRNA level. Moreover, the enzymatic activity of RNase E rapidly increased as well, although the protein stability decreased. This unique response was underpinned by the increased accumulation of full-length rne mRNA caused by the stabilization of its 5' UTR and suppression of premature transcriptional termination, but not by an increased transcription rate. Mapping of RNA 3' ends and in vitro cleavage assays revealed that RNase E cleaves within a stretch of six consecutive uridine residues within the rne 5' UTR, indicating autoregulation. These observations suggest that RNase E in cyanobacteria contributes to reshaping the transcriptome during the UV stress response and that its required activity level is secured at the RNA level despite the enhanced turnover of the protein.
Keywords: cyanobacteria; protein turnover; ribonuclease; stress response.
© 2023 The Authors. mLife published by John Wiley & Sons Australia, Ltd. on behalf of Institute of Microbiology, Chinese Academy of Sciences.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





Similar articles
-
An evolutionarily conserved RNA stem-loop functions as a sensor that directs feedback regulation of RNase E gene expression.Genes Dev. 2000 May 15;14(10):1249-60. Genes Dev. 2000. PMID: 10817759 Free PMC article.
-
Dark-induced mRNA instability involves RNase E/G-type endoribonuclease cleavage at the AU-box and SD sequences in cyanobacteria.Mol Genet Genomics. 2007 Sep;278(3):331-46. doi: 10.1007/s00438-007-0254-9. Epub 2007 Jul 28. Mol Genet Genomics. 2007. PMID: 17661085
-
RNase E autoregulates its synthesis by controlling the degradation rate of its own mRNA in Escherichia coli: unusual sensitivity of the rne transcript to RNase E activity.Genes Dev. 1995 Jan 1;9(1):84-96. doi: 10.1101/gad.9.1.84. Genes Dev. 1995. PMID: 7530223
-
Using the power of genetic suppressors to probe the essential functions of RNase E.Curr Genet. 2016 Feb;62(1):53-7. doi: 10.1007/s00294-015-0510-1. Epub 2015 Aug 1. Curr Genet. 2016. PMID: 26232079 Review.
-
"Life is short, and art is long": RNA degradation in cyanobacteria and model bacteria.mLife. 2022 Mar 24;1(1):21-39. doi: 10.1002/mlf2.12015. eCollection 2022 Mar. mLife. 2022. PMID: 38818322 Free PMC article. Review.
Cited by
-
Alkane biosynthesis gene expression and its increased production in recombinant cyanobacteria.FEBS Open Bio. 2025 Jun;15(6):949-962. doi: 10.1002/2211-5463.70009. Epub 2025 Mar 6. FEBS Open Bio. 2025. PMID: 40047142 Free PMC article.
-
A conserved protein inhibitor brings under check the activity of RNase E in cyanobacteria.Nucleic Acids Res. 2024 Jan 11;52(1):404-419. doi: 10.1093/nar/gkad1094. Nucleic Acids Res. 2024. PMID: 38000383 Free PMC article.
-
The RRM domain-containing protein Rbp3 interacts with ribosomes and the 3' ends of mRNAs encoding photosynthesis proteins.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2506275122. doi: 10.1073/pnas.2506275122. Epub 2025 Jun 24. Proc Natl Acad Sci U S A. 2025. PMID: 40553498
-
The role of the 5' sensing function of ribonuclease E in cyanobacteria.RNA Biol. 2024 Jan;21(1):1-18. doi: 10.1080/15476286.2024.2328438. Epub 2024 Mar 12. RNA Biol. 2024. PMID: 38469716 Free PMC article.
References
-
- Coburn GA, Mackie GA. Degradation of mRNA in Escherichia coli: an old problem with some new twists. Prog Nucleic Acid Res Mol Biol. 1999;62:55–108. - PubMed
-
- Mackie GA. RNase E: at the interface of bacterial RNA processing and decay. Nat Rev Microbiol. 2013;11:45–57. - PubMed
-
- Ehretsmann CP, Carpousis AJ, Krisch HM. Specificity of Escherichia coli endoribonuclease RNase E: in vivo and in vitro analysis of mutants in a bacteriophage T4 mRNA processing site. Genes Dev. 1992;6:149–59. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
