Selective and brain-penetrant ACSS2 inhibitors target breast cancer brain metastatic cells
- PMID: 38818373
- PMCID: PMC11137182
- DOI: 10.3389/fphar.2024.1394685
Selective and brain-penetrant ACSS2 inhibitors target breast cancer brain metastatic cells
Abstract
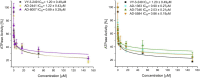
Breast cancer brain metastasis (BCBM) typically results in an end-stage diagnosis and is hindered by a lack of brain-penetrant drugs. Tumors in the brain rely on the conversion of acetate to acetyl-CoA by the enzyme acetyl-CoA synthetase 2 (ACSS2), a key regulator of fatty acid synthesis and protein acetylation. Here, we used a computational pipeline to identify novel brain-penetrant ACSS2 inhibitors combining pharmacophore-based shape screen methodology with absorption, distribution, metabolism, and excretion (ADME) property predictions. We identified compounds AD-5584 and AD-8007 that were validated for specific binding affinity to ACSS2. Treatment of BCBM cells with AD-5584 and AD-8007 leads to a significant reduction in colony formation, lipid storage, acetyl-CoA levels and cell survival in vitro. In an ex vivo brain-tumor slice model, treatment with AD-8007 and AD-5584 reduced pre-formed tumors and synergized with irradiation in blocking BCBM tumor growth. Treatment with AD-8007 reduced tumor burden and extended survival in vivo. This study identifies selective brain-penetrant ACSS2 inhibitors with efficacy towards breast cancer brain metastasis.
Keywords: ACSS2; acetate; acetyl-CoA; brain metastasis; breast cancer; cancer; computational-aided drug design font: italic formatted: left; metabolism.
Copyright © 2024 Esquea, Ciraku, Young, Merzy, Talarico, Ahmed, Karuppiah, Ramesh, Chatoff, Crispim, Rashad, Cocklin, Snyder, Beld, Simone, Reginato and Dick.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

