Quantitative evaluation of the medicine innovation policy in China: based on the PMC-Index model
- PMID: 38818446
- PMCID: PMC11137217
- DOI: 10.3389/fpubh.2024.1403320
Quantitative evaluation of the medicine innovation policy in China: based on the PMC-Index model
Abstract
Introduction: Medicine innovation is crucial in promoting the sustainable development of medicine undertakings, which has significant economic and social benefits. China is the main force in global medicine consumption, with a huge demand for innovative medicines. Thus, the Chinese government releases a series of policies aimed at providing scientific and reasonable guidance for medicine innovation. However, there is inadequate quantitative evaluation and comparison of various medicine innovation policies in the existing studies.
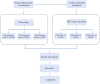
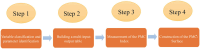
Methods: This paper adopts the approach of text mining and the Policy Modeling Consistency Index (PMC-Index) model to construct an evaluation system and then quantitatively evaluates and compares the traditional Chinese medicine innovation policies (TCMIPs), the biological medicine innovation policies (BMIPs), and the multiple medicine innovation policies (MMIPs) in China.
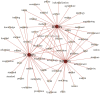
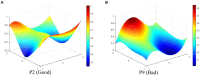
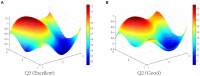
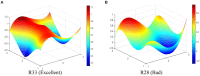
Results: The results indicate that: (1) The three types of drug innovation policies have similarities in content and goal through comparative analysis of high-frequency words, while they also have their own characteristics. (2) The average PMC-Index of 29 TCMIPs is 5.77, which has the highest policy bad rate (21%); the average PMC-Index of 12 BMIPs is 6.21, which has the highest policy good rate (92%); moreover, the average PMC-Index of 35 MMIPs is 6.06, which has the highest policy excellence rate (26%). (3) The BMIPs, MMIPs, and TCMIPs have similar scores on policy object, policy orientation, policy timeliness, policy evaluation, and policy accessibility, while they differ significantly mainly on policy nature, incentive method, policy function, policy issuing agency, and policy instrument.
Discussion: This study contributes to a comprehensive understanding of medicine innovation policies in China, in order to provide theoretical support for future policy formulation and optimization in the medicine industry. Moreover, we expand the application scenarios of policy diffusion theory.
Keywords: China; PMC-Index model; medicine innovation policy; policy formulation; quantitative evaluation.
Copyright © 2024 Guo, Qi and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Pe M, Alanya A, Falk RS, Amdal CD, Bjordal K, Chang J, et al. . Setting international standards in analyzing patient- reported outcomes and quality of life endpoints in cancer clinical trials-innovative medicines initiative (SISAQOL-IMI) stakeholder views, objectives, and procedures. Lancet Oncol. (2023) 24:E270–83. doi: 10.1016/s1470-2045(23)00157-2, PMID: - DOI - PubMed
-
- Chen Y. How to boost long-term confidence in China’s innovative drugs. China Reform. (2023) 1:59–65.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

