Organokines and liver enzymes in adolescent girls with polycystic ovary syndrome during randomized treatments
- PMID: 38818508
- PMCID: PMC11137167
- DOI: 10.3389/fendo.2024.1325230
Organokines and liver enzymes in adolescent girls with polycystic ovary syndrome during randomized treatments
Abstract
Introduction: Polycystic ovary syndrome (PCOS) is often associated with metabolic-associated fatty liver disease (MAFLD). MAFLD has been associated with altered hepatic function, systemic dysmetabolism, and abnormal circulating levels of signaling molecules called organokines. Here, we assessed the effects of two randomized treatments on a set of organokines in adolescent girls with PCOS and without obesity, and report the associations with circulating biomarkers of liver damage, which were assessed longitudinally in the aforementioned studies as safety markers.
Materials and methods: Liver enzymes [aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT)] were assessed as safety markers in previous randomized pilot studies comparing the effects of an oral contraceptive (OC) with those of a low-dose combination of spironolactone-pioglitazone-metformin (spiomet) for 1 year. As a post hoc endpoint, the organokines fibroblast growth factor-21 (FGF21), diazepam-binding protein-1 (DBI), and meteorin-like protein (METRNL) were assessed by ELISA after 6 months of OC (N = 26) or spiomet (N = 28). Auxological, endocrine-metabolic, body composition (using DXA), and abdominal fat partitioning (using MRI) were also evaluated. Healthy, age-matched adolescent girls (N = 17) served as controls.
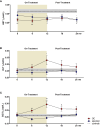
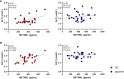
Results: Circulating ALT and GGT levels increased during OC treatment and returned to baseline concentrations in the post-treatment phase; in contrast, spiomet treatment elicited no detectable changes in ALT and GGT concentrations. In relation to organokines after 6 months of treatment, (1) FGF21 levels were significantly higher in PCOS adolescents than in control girls; (2) DBI levels were lower in OC-treated girls than in controls and spiomet-treated girls; and (3) no differences were observed in METRNL concentrations between PCOS girls and controls. Serum ALT and GGT levels were directly correlated with circulating METRNL levels only in OC-treated girls (R = 0.449, P = 0.036 and R = 0.552, P = 0.004, respectively).
Conclusion: The on-treatment increase in ALT and GGT levels occurring only in OC-treated girls is associated with circulating METRNL levels, suggesting enhanced METRNL synthesis as a reaction to the hepatic changes elicited by OC treatment.
Clinical trial registration: https://doi.org, identifiers 10.1186/ISRCTN29234515, 10.1186/ISRCTN11062950.
Keywords: METRNL; PCOS; liver enzymes; metformin; oral contraceptives; organokines; pioglitazone; spironolactone.
Copyright © 2024 Garcia-Beltran, Peyrou, Navarro-Gascon, López-Bermejo, de Zegher, Villarroya and Ibáñez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The relative deficit of GDF15 in adolescent girls with PCOS can be changed into an abundance that reduces liver fat.Sci Rep. 2021 Mar 29;11(1):7018. doi: 10.1038/s41598-021-86317-9. Sci Rep. 2021. PMID: 33782413 Free PMC article. Clinical Trial.
-
Low Circulating Levels of miR-451a in Girls with Polycystic Ovary Syndrome: Different Effects of Randomized Treatments.J Clin Endocrinol Metab. 2020 Mar 1;105(3):dgz204. doi: 10.1210/clinem/dgz204. J Clin Endocrinol Metab. 2020. PMID: 31730174 Clinical Trial.
-
Towards a circulating marker of hepato-visceral fat excess: S100A4 in adolescent girls with polycystic ovary syndrome - Evidence from randomized clinical trials.Pediatr Obes. 2019 May;14(5):e12500. doi: 10.1111/ijpo.12500. Epub 2019 Jan 17. Pediatr Obes. 2019. PMID: 30653851 Clinical Trial.
-
Hepatic manifestations of women with polycystic ovary syndrome.Best Pract Res Clin Obstet Gynaecol. 2016 Nov;37:119-128. doi: 10.1016/j.bpobgyn.2016.03.003. Epub 2016 Apr 6. Best Pract Res Clin Obstet Gynaecol. 2016. PMID: 27107966 Review.
-
The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: An up-to date systematic review and meta-analysis of randomized controlled trials.Pharmacol Res. 2020 Sep;159:104799. doi: 10.1016/j.phrs.2020.104799. Epub 2020 Apr 8. Pharmacol Res. 2020. PMID: 32278041
Cited by
-
Type 2 Diabetes Mellitus and Cardiometabolic Prospects: A Rapid Narrative Review.Cureus. 2024 Jul 30;16(7):e65808. doi: 10.7759/cureus.65808. eCollection 2024 Jul. Cureus. 2024. PMID: 39092382 Free PMC article. Review.
-
Comparative Efficacy of Metformin and Combined Oral Contraceptives in the Management of Adolescent Polycystic Ovary Syndrome: A Systematic Review of Randomized Controlled Trials.Cureus. 2025 May 10;17(5):e83850. doi: 10.7759/cureus.83850. eCollection 2025 May. Cureus. 2025. PMID: 40491634 Free PMC article. Review.
-
Research on Fibroblast Growth Factor 21 and Its Relationship to Patients with Polycystic Ovary Syndrome and Obesity.Diabetes Metab Syndr Obes. 2025 Jun 26;18:2029-2039. doi: 10.2147/DMSO.S519236. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 40589499 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

