Water channels in the brain and spinal cord-overview of the role of aquaporins in traumatic brain injury and traumatic spinal cord injury
- PMID: 38818518
- PMCID: PMC11137310
- DOI: 10.3389/fncel.2024.1414662
Water channels in the brain and spinal cord-overview of the role of aquaporins in traumatic brain injury and traumatic spinal cord injury
Abstract
Knowledge about the mechanisms underlying the fluid flow in the brain and spinal cord is essential for discovering the mechanisms implicated in the pathophysiology of central nervous system diseases. During recent years, research has highlighted the complexity of the fluid flow movement in the brain through a glymphatic system and a lymphatic network. Less is known about these pathways in the spinal cord. An important aspect of fluid flow movement through the glymphatic pathway is the role of water channels, especially aquaporin 1 and 4. This review provides an overview of the role of these aquaporins in brain and spinal cord, and give a short introduction to the fluid flow in brain and spinal cord during in the healthy brain and spinal cord as well as during traumatic brain and spinal cord injury. Finally, this review gives an overview of the current knowledge about the role of aquaporins in traumatic brain and spinal cord injury, highlighting some of the complexities and knowledge gaps in the field.
Keywords: aquaporins; glymphatic system; lymphatic network; traumatic brain injury; traumatic spinal cord injury.
Copyright © 2024 Overgaard Wichmann, Hedegaard Højsager and Hasager Damkier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
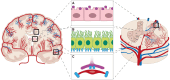
Similar articles
-
A Brief Overview of the Cerebrospinal Fluid System and Its Implications for Brain and Spinal Cord Diseases.Front Hum Neurosci. 2022 Jan 21;15:737217. doi: 10.3389/fnhum.2021.737217. eCollection 2021. Front Hum Neurosci. 2022. PMID: 35126070 Free PMC article. Review.
-
Expression of aquaporin water channels in mouse spinal cord.Neuroscience. 2004;127(3):685-93. doi: 10.1016/j.neuroscience.2004.03.016. Neuroscience. 2004. PMID: 15283967
-
The glymphatic pathway in neurological disorders.Lancet Neurol. 2018 Nov;17(11):1016-1024. doi: 10.1016/S1474-4422(18)30318-1. Lancet Neurol. 2018. PMID: 30353860 Free PMC article. Review.
-
Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy.Neurosci Biobehav Rev. 2018 Jan;84:316-324. doi: 10.1016/j.neubiorev.2017.08.016. Epub 2017 Aug 30. Neurosci Biobehav Rev. 2018. PMID: 28859995 Review.
-
The Role of Aquaporins in Spinal Cord Injury.Cells. 2023 Jun 23;12(13):1701. doi: 10.3390/cells12131701. Cells. 2023. PMID: 37443735 Free PMC article. Review.
Cited by
-
Meningeal lymphatic drainage: novel insights into central nervous system disease.Signal Transduct Target Ther. 2025 May 5;10(1):142. doi: 10.1038/s41392-025-02177-z. Signal Transduct Target Ther. 2025. PMID: 40320416 Free PMC article. Review.
-
The Crucial Role of the Blood-Brain Barrier in Neurodegenerative Diseases: Mechanisms of Disruption and Therapeutic Implications.J Clin Med. 2025 Jan 9;14(2):386. doi: 10.3390/jcm14020386. J Clin Med. 2025. PMID: 39860392 Free PMC article. Review.
-
A budget for brain metabolic water production by glucose catabolism during rest, rises in activity and sleep.Fluids Barriers CNS. 2025 May 6;22(1):44. doi: 10.1186/s12987-025-00647-8. Fluids Barriers CNS. 2025. PMID: 40329309 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

