QPGx-CARES: Qatar pharmacogenetics clinical applications and research enhancement strategies
- PMID: 38818903
- PMCID: PMC11140449
- DOI: 10.1111/cts.13800
QPGx-CARES: Qatar pharmacogenetics clinical applications and research enhancement strategies
Abstract
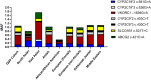
Pharmacogenetic (PGx)-informed medication prescription is a cutting-edge genomic application in contemporary medicine, offering the potential to overcome the conventional "trial-and-error" approach in drug prescription. The ability to use an individual's genetic profile to predict drug responses allows for personalized drug and dosage selection, thereby enhancing the safety and efficacy of treatments. However, despite significant scientific and clinical advancements in PGx, its integration into routine healthcare practices remains limited. To address this gap, the Qatar Genome Program (QGP) has embarked on an ambitious initiative known as QPGx-CARES (Qatar Pharmacogenetics Clinical Applications and Research Enhancement Strategies), which aims to set a roadmap for optimizing PGx research and clinical implementation on a national scale. The goal of QPGx-CARES initiative is to integrate PGx testing into clinical settings with the aim of improving patient health outcomes. In 2022, QGP initiated several implementation projects in various clinical settings. These projects aimed to evaluate the clinical utility of PGx testing, gather valuable insights into the effective dissemination of PGx data to healthcare professionals and patients, and identify the gaps and the challenges for wider adoption. QPGx-CARES strategy aimed to integrate evidence-based PGx findings into clinical practice, focusing on implementing PGx testing for cardiovascular medications, supported by robust scientific evidence. The current initiative sets a precedent for the nationwide implementation of precision medicine across diverse clinical domains.
© 2024 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures


References
-
- Pardiñas AF, Owen MJ, Walters JT. Pharmacogenomics: a road ahead for precision medicine in psychiatry. Neuron. 2021;109:3914‐3929. - PubMed
-
- Hjemås BJ, Bøvre K, Bjerknes K, Mathiesen L, Mellingsæter MCR, Molden E. Implementation of pharmacogenetic testing in medication reviews in a hospital setting. Br J Clin Pharmacol. 2023;89:3116‐3125. - PubMed
-
- Matey ET, Ragan AK, Oyen LJ, et al. Nine‐gene pharmacogenomics profile service: the Mayo Clinic experience. Pharmacogenomics J. 2022;22:69‐74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

