Lorlatinib Versus Crizotinib in Patients With Advanced ALK-Positive Non-Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study
- PMID: 38819031
- PMCID: PMC11458101
- DOI: 10.1200/JCO.24.00581
Lorlatinib Versus Crizotinib in Patients With Advanced ALK-Positive Non-Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study
Abstract
Purpose: Lorlatinib improved progression-free survival (PFS) and intracranial activity versus crizotinib in patients with previously untreated, advanced, ALK-positive non-small cell lung cancer (NSCLC) in the phase III CROWN study. Here, we report long-term outcomes from CROWN after 5 years of follow-up.
Methods: Two hundred ninety-six patients with ALK-positive NSCLC were randomly assigned 1:1 to receive lorlatinib 100 mg once daily (n = 149) or crizotinib 250 mg twice daily (n = 147). This post hoc analysis presents updated investigator-assessed efficacy outcomes, safety, and biomarker analyses.
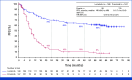
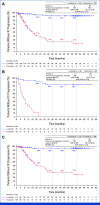
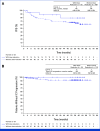
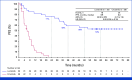
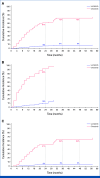
Results: With a median follow-up for PFS of 60.2 and 55.1 months, respectively, median PFS was not reached (NR [95% CI, 64.3 to NR]) with lorlatinib and 9.1 months (95% CI, 7.4 to 10.9) with crizotinib (hazard ratio [HR], 0.19 [95% CI, 0.13 to 0.27]); 5-year PFS was 60% (95% CI, 51 to 68) and 8% (95% CI, 3 to 14), respectively. Median time to intracranial progression was NR (95% CI, NR to NR) with lorlatinib and 16.4 months (95% CI, 12.7 to 21.9) with crizotinib (HR, 0.06 [95% CI, 0.03 to 0.12]). Safety profile was consistent with that in prior analyses. Emerging new ALK resistance mutations were not detected in circulating tumor DNA collected at the end of lorlatinib treatment.
Conclusion: After 5 years of follow-up, median PFS has yet to be reached in the lorlatinib group, corresponding to the longest PFS ever reported with any single-agent molecular targeted treatment in advanced NSCLC and across all metastatic solid tumors. These results coupled with prolonged intracranial efficacy and absence of new safety signals represent an unprecedented outcome for patients with advanced ALK-positive NSCLC and set a new benchmark for targeted therapies in cancer.
Trial registration: ClinicalTrials.gov NCT03052608.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


Comment in
-
New Benchmark for Targeted Therapies in Lung Cancer: Median Progression-Free Survival for Lorlatinib in Advanced ALK+ Non-Small Cell Lung Cancer Surpasses 5 years.J Clin Oncol. 2024 Oct 10;42(29):3383-3386. doi: 10.1200/JCO.24.01147. Epub 2024 Sep 4. J Clin Oncol. 2024. PMID: 39231392 Free PMC article.
-
Lorlatinib in frontline treatment of advanced ALK-positive non-small cell lung cancer: a highly efficient new standard of care but still challenging to manage.Transl Lung Cancer Res. 2025 Jan 24;14(1):1-6. doi: 10.21037/tlcr-24-903. Epub 2025 Jan 17. Transl Lung Cancer Res. 2025. PMID: 39958226 Free PMC article. No abstract available.
References
-
- Planchard D, Popat S, Kerr K, et al. : Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv192-iv237, 2018. (suppl 4) - PubMed
-
- Johnson TW, Richardson PF, Bailey S, et al. : Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h] [2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 57:4720-4744, 2014 - PubMed
-
- Shaw AT, Bauer TM, de Marinis F, et al. : First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 383:2018-2029, 2020 - PubMed
-
- Solomon BJ, Bauer TM, Mok TSK, et al. : Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: Updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med 11:354-366, 2023 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

