Human coronavirus OC43 infection remodels connexin 43-mediated gap junction intercellular communication in vitro
- PMID: 38819132
- PMCID: PMC11264776
- DOI: 10.1128/jvi.00478-24
Human coronavirus OC43 infection remodels connexin 43-mediated gap junction intercellular communication in vitro
Abstract
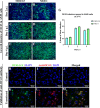
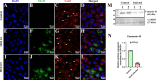
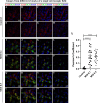
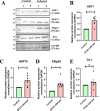
β-coronaviruses cause acute infection in the upper respiratory tract, resulting in various symptoms and clinical manifestations. OC43 is a human β-coronavirus that induces mild clinical symptoms and can be safely studied in the BSL2 laboratory. Due to its low risk, OC43 can be a valuable and accessible model for understanding β-coronavirus pathogenesis. One potential target for limiting virus infectivity could be gap junction-mediated communication. This study aims to unveil the status of cell-to-cell communications through gap junctions in human β-coronavirus infection. Infection with OC43 leads to reduced expression of Cx43 in A549, a lung epithelial carcinoma cell line. Infection with this virus also shows a significant ER and oxidative stress increase. Internal localization of Cx43 is observed post-OC43 infection in the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) region, which impairs the gap junction communication between two adjacent cells, confirmed by Lucifer yellow dye transfer assay. It also affects hemichannel formation, as depicted by the EtBr uptake assay. Impairment of Cx43 trafficking and the ability to form hemichannels and functional GJIC are hampered by virus-induced Golgi apparatus disruption. Altogether, these results suggest that several physiological changes accompany OC43 infection in A549 cells and can be considered an appropriate model system for understanding the differences in gap junction communication post-viral infections. This model system can provide valuable insights for developing therapies against human β-coronavirus infections.IMPORTANCEThe enduring impact of the recent SARS-CoV-2 pandemic underscores the importance of studying human β-coronaviruses, advancing our preparedness for future coronavirus infections. As SARS-CoV-2 is highly infectious, another human β-coronavirus OC43 can be considered an experimental model. One of the crucial pathways that can be considered is gap junction communication, as it is vital for cellular homeostasis. Our study seeks to understand the changes in Cx43-mediated cell-to-cell communication during human β-coronavirus OC43 infection. In vitro studies showed downregulation of the gap junction protein Cx43 and upregulation of the endoplasmic reticulum and oxidative stress markers post-OC43 infection. Furthermore, HCoV-OC43 infection causes reduced Cx43 trafficking, causing impairment of functional hemichannel and GJIC formation by virus-mediated Golgi apparatus disruption. Overall, this study infers that OC43 infection reshapes intercellular communication, suggesting that this pathway may be a promising target for designing highly effective therapeutics against human coronaviruses by regulating Cx43 expression.
Keywords: Golgi apparatus disruption; OC43; connexin 43; gap junction communication; human β-coronavirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mouse Hepatitis Virus Infection Remodels Connexin43-Mediated Gap Junction Intercellular Communication In Vitro and In Vivo.J Virol. 2015 Dec 16;90(5):2586-99. doi: 10.1128/JVI.02420-15. J Virol. 2015. PMID: 26676788 Free PMC article.
-
Amyloid-β regulates gap junction protein connexin 43 trafficking in cultured primary astrocytes.J Biol Chem. 2020 Oct 30;295(44):15097-15111. doi: 10.1074/jbc.RA120.013705. Epub 2020 Aug 31. J Biol Chem. 2020. PMID: 32868453 Free PMC article.
-
Regulatory role of endoplasmic reticulum resident chaperone protein ERp29 in anti-murine β-coronavirus host cell response.J Biol Chem. 2023 Feb;299(2):102836. doi: 10.1016/j.jbc.2022.102836. Epub 2022 Dec 23. J Biol Chem. 2023. PMID: 36572185 Free PMC article.
-
Trafficking highways to the intercalated disc: new insights unlocking the specificity of connexin 43 localization.Cell Commun Adhes. 2014 Feb;21(1):43-54. doi: 10.3109/15419061.2013.876014. Cell Commun Adhes. 2014. PMID: 24460200 Free PMC article. Review.
-
Analysis of the function and therapeutic strategy of connexin 43 from its subcellular localization.Biochimie. 2024 Mar;218:1-7. doi: 10.1016/j.biochi.2023.08.011. Epub 2023 Aug 22. Biochimie. 2024. PMID: 37611889 Review.
Cited by
-
Modulation of connexin 43 in viral infections.Tumour Virus Res. 2024 Dec;18:200296. doi: 10.1016/j.tvr.2024.200296. Epub 2024 Nov 8. Tumour Virus Res. 2024. PMID: 39522757 Free PMC article. Review.
-
Viral Infection and Connexin Dysfunction in the Heart.Curr Cardiol Rep. 2025 Mar 27;27(1):76. doi: 10.1007/s11886-025-02227-6. Curr Cardiol Rep. 2025. PMID: 40146392 Free PMC article. Review.
References
-
- Li S, Armstrong N, Zhao H, Cruz-cosme R, Yang H, Zhong C, Fu W, Wang W, Yang D, Xia N, Cheng T, Tang Q. 2022. Zika virus infection downregulates connexin 43, disrupts the cardiomyocyte gap junctions and induces heart diseases in A129 mice. J Virol 96:1–18. doi:10.1128/jvi.01373-22 - DOI - PMC - PubMed
-
- Khan Z, Yaiw K-C, Wilhelmi V, Lam H, Rahbar A, Stragliotto G, Söderberg-Nauclér C. 2014. Human cytomegalovirus immediate early proteins promote degradation of connexin 43 and disrupt gap junction communication: Implications for a role in gliomagenesis. Carcinogenesis 35:145–154. doi:10.1093/carcin/bgt292 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

