Prolonged cytopenias after immune effector cell therapy and lymphodepletion in patients with leukemia, lymphoma and solid tumors
- PMID: 38819365
- PMCID: PMC11344664
- DOI: 10.1016/j.jcyt.2024.04.075
Prolonged cytopenias after immune effector cell therapy and lymphodepletion in patients with leukemia, lymphoma and solid tumors
Abstract
Background aims: The success of chimeric antigen receptor (CAR) T-cell therapy in treating B-cell malignancies has led to the evaluation of CAR T-cells targeting a variety of other malignancies. Although the efficacy of CAR T-cells is enhanced when administered post-lymphodepleting chemotherapy, this can trigger bone marrow suppression and sustained cytopenia after CD19.CAR T-cell therapy. Additionally, systemic inflammation associated with CAR T-cell activity may contribute to myelosuppression. Cytopenias, such as neutropenia and thrombocytopenia, elevate the risk of severe infections and bleeding, respectively. However, data on the incidence of prolonged cytopenias after immune effector therapy in the solid tumor context remain limited.
Objective: We compared the incidence of prolonged cytopenias after immune effector therapy including genetically modified T-cells, virus-specific T-cells (VSTs) and NKT-cells, as well non-gene-modified VSTs for leukemia, lymphoma, and solid tumors (ST) to identify associated risk factors.
Methods: A retrospective analysis was conducted of 112 pediatric and adult patients with relapsed and/or refractory cancers who received lymphodepleting chemotherapy followed by immune effector therapy. Patients treated with 13 distinct immune effector cell therapies through 11 single-center clinical trials and 2 commercial products over a 6-year period were categorized into 3 types of malignancies: leukemia, lymphoma and ST. We obtained baseline patient characteristics and adverse events data for each participant, and tracked neutrophil and platelet counts following lymphodepletion.
Results: Of 112 patients, 104 (92.9%) experienced cytopenias and 88 (79%) experienced severe cytopenias. Patients with leukemia experienced significantly longer durations of severe neutropenia (median duration of 14 days) compared with patients with lymphoma (7 days) or ST (11 days) (P = 0.002). Patients with leukemia also had a higher incidence of severe thrombocytopenia (74.1%), compared with lymphoma (46%, P = 0.03) and ST (14.3%, P < 0.0001). Prolonged cytopenias were significantly associated with disease type (63% of patients with leukemia, 44% of patients with lymphoma, and 22.9% of patients with ST, P = 0.006), prior hematopoietic stem cell transplant (HSCT) (66.7% with prior HSCT versus 38.3% without prior HSCT, P = 0.039), and development of immune effector cell-associated neurotoxicity syndrome (ICANS) (75% with ICANS versus 38% without ICANS, P = 0.027). There was no significant association between prolonged cytopenias and cytokine release syndrome.
Conclusions: Immune effector recipients often experience significant cytopenias due to marrow suppression following lymphodepletion regardless of disease, but prolonged severe cytopenias are significantly less common after treatment of patients with lymphoma and solid tumors.
Keywords: CAR T cells; cytopenia; hematologic toxicity; immune effector cells.
Published by Elsevier Inc.
Figures
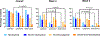
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

