Early- to mid-gestational testosterone excess leads to adverse cardiac outcomes in postpartum sheep
- PMID: 38819385
- PMCID: PMC11687963
- DOI: 10.1152/ajpheart.00763.2023
Early- to mid-gestational testosterone excess leads to adverse cardiac outcomes in postpartum sheep
Abstract
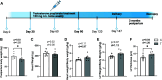
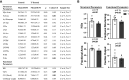
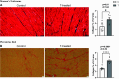
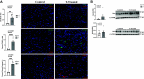
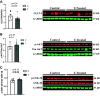
Cardiovascular dysfunctions complicate 10-20% of pregnancies, increasing the risk for postpartum mortality. Various gestational insults, including preeclampsia are reported to be associated with adverse maternal cardiovascular outcomes. One such insult, gestational hyperandrogenism increases the risk for preeclampsia and other gestational morbidities but its impact on postpartum maternal health is not well known. We hypothesize that gestational hyperandrogenism such as testosterone (T) excess will adversely impact the maternal heart in the postpartum period. Pregnant ewes were injected with T propionate from day 30 to day 90 of gestation (term 147 days). Three months postpartum, echocardiograms, plasma cytokine profiles, cardiac morphometric, and molecular analysis were conducted [control (C) n = 6, T-treated (T) n = 7 number of animals]. Data were analyzed by two-tailed Student's t test and Cohen's effect size (d) analysis. There was a nonsignificant large magnitude decrease in cardiac output (7.64 ± 1.27 L/min vs. 10.19 ± 1.40, P = 0.22, d = 0.81) and fractional shortening in the T ewes compared with C (35.83 ± 2.33% vs. 41.50 ± 2.84, P = 0.15, d = 0.89). T treatment significantly increased 1) left ventricle (LV) weight-to-body weight ratio (2.82 ± 0.14 g/kg vs. 2.46 ± 0.08) and LV thickness (14.56 ± 0.52 mm vs. 12.50 ± 0.75), 2) proinflammatory marker [tumor necrosis factor-alpha (TNF-α)] in LV (1.66 ± 0.35 vs. 1.06 ± 0.18), 3) LV collagen (Masson's Trichrome stain: 3.38 ± 0.35 vs. 1.49 ± 0.15 and Picrosirius red stain: 5.50 ± 0.32 vs. 3.01 ± 0.23), 4) markers of LV apoptosis, including TUNEL (8.3 ± 1.1 vs. 0.9 ± 0.18), bcl-2-associated X protein (Bax)+-to-b-cell lymphoma 2 (Bcl2)+ ratio (0.68 ± 0.30 vs. 0.13 ± 0.02), and cleaved caspase 3 (15.4 ± 1.7 vs. 4.4 ± 0.38). These findings suggest that gestational testosterone excess adversely programs the maternal LV, leading to adverse structural and functional consequences in the postpartum period.NEW & NOTEWORTHY Using a sheep model of human translational relevance, this study provides evidence that excess gestational testosterone exposure such as that seen in hyperandrogenic disorders adversely impacts postpartum maternal hearts.
Keywords: cardiovascular; hyperandrogenism; maternal; ovine; postpartum.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


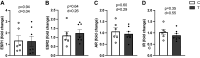
Similar articles
-
Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes.Cochrane Database Syst Rev. 2015 Dec 15;2015(12):CD009916. doi: 10.1002/14651858.CD009916.pub2. Cochrane Database Syst Rev. 2015. PMID: 26671418 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes.Cochrane Database Syst Rev. 2018 Jul 24;7(7):CD010564. doi: 10.1002/14651858.CD010564.pub2. Cochrane Database Syst Rev. 2018. PMID: 30039871 Free PMC article.
-
Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants.Cochrane Database Syst Rev. 2018 Jan 5;1(1):CD012910. doi: 10.1002/14651858.CD012910. Cochrane Database Syst Rev. 2018. PMID: 29303230 Free PMC article.
-
Iodine supplementation for women during the preconception, pregnancy and postpartum period.Cochrane Database Syst Rev. 2017 Mar 5;3(3):CD011761. doi: 10.1002/14651858.CD011761.pub2. Cochrane Database Syst Rev. 2017. PMID: 28260263 Free PMC article.
References
-
- Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, Fat DM, Boerma T, Temmerman M, Mathers C, Say L; United Nations Maternal Mortality Estimation Inter-Agency Group collaborators and technical advisory group. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 387: 462–474, 2016. doi: 10.1016/S0140-6736(15)00838-7. - DOI - PMC - PubMed
-
- Lameijer H, Schutte JM, Schuitemaker NWE, van Roosmalen JJM, Pieper PG, Dutch MM, Morbidity C; Dutch Maternal Mortality and Morbidity Committee. Maternal mortality due to cardiovascular disease in the Netherlands: a 21-year experience. Neth Heart J 28: 27–36, 2020. doi: 10.1007/s12471-019-01340-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

