Human 3D Ovarian Cancer Models Reveal Malignant Cell-Intrinsic and -Extrinsic Factors That Influence CAR T-cell Activity
- PMID: 38819641
- PMCID: PMC11292204
- DOI: 10.1158/0008-5472.CAN-23-3007
Human 3D Ovarian Cancer Models Reveal Malignant Cell-Intrinsic and -Extrinsic Factors That Influence CAR T-cell Activity
Abstract
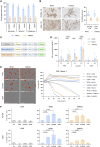
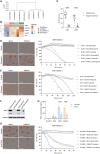
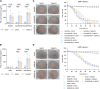
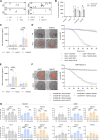
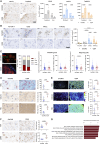
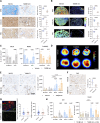
In vitro preclinical testing of chimeric antigen receptor (CAR) T cells is mostly carried out in monolayer cell cultures. However, alternative strategies are needed to take into account the complexity and the effects of the tumor microenvironment. Here, we describe the modulation of CAR T-cell activity by malignant cells and fibroblasts in human three-dimensional (3D) in vitro cell models of increasing complexity. In models combining mucin-1 (MUC1) and TnMUC1 CAR T cells with human high-grade serous ovarian cancer cell spheroids, malignant cell-intrinsic resistance to CAR T-cell killing was due to defective death receptor signaling involving TNFα. Adding primary human fibroblasts to spheroids unexpectedly increased the ability of CAR T cells to kill resistant malignant cells as CCL2 produced by fibroblasts activated CCR2/4+ CAR T cells. However, culturing malignant cells and fibroblasts in collagen gels engendered production of a dense extracellular matrix that impeded CAR T-cell activity in a TGFβ-dependent manner. A vascularized microfluidic device was developed that allowed CAR T cells to flow through the vessels and penetrate the gels in a more physiological way, killing malignant cells in a TNFα-dependent manner. Complex 3D human cell models may provide an efficient way of screening multiple cytotoxic human immune cell constructs while also enabling evaluation of mechanisms of resistance involving cell-cell and cell-matrix interactions, thus accelerating preclinical research on cytotoxic immune cell therapies in solid tumors. Significance: Three-dimensional in vitro models of increasing complexity uncover mechanisms of resistance to CAR T cells in solid tumors, which could help accelerate development of improved CAR T-cell constructs.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J.D. Joy reports grants from GSK during the conduct of the study. R. Manchanda reports grants from Barts Charity during the conduct of the study; grants from GSK, Yorkshire Cancer Research, Rosetrees Trust, and North East London Cancer Alliance, and other support from AstraZeneca, MSD, and EGL outside the submitted work. F.R. Balkwill reports grants from GSK during the conduct of the study and personal fees from iOmx therapeutics outside the submitted work. No disclosures were reported by the other authors.
Figures

References
-
- Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard I-L, et al. . Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Obstet Gynecol Surv 2021;76:673–75. - PubMed
-
- Drew Y, Penson RT, O’Malley DM, Kim J-W, Zimmermann S, Roxburgh P, et al. . 814MO Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol 2020;31(Suppl 4):S615–6.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

