The Effects of a Dietary Supplement (PediaFlù) Plus Standard of Care in Children With Acute Tonsillopharyngitis/Rhinopharyngitis: Protocol for a Randomized Controlled Trial
- PMID: 38819917
- PMCID: PMC11179036
- DOI: 10.2196/53703
The Effects of a Dietary Supplement (PediaFlù) Plus Standard of Care in Children With Acute Tonsillopharyngitis/Rhinopharyngitis: Protocol for a Randomized Controlled Trial
Abstract
Background: A dietary supplement containing Pelargonium sidoides extract, propolis, zinc, and honey has been recently developed and proven to be an effective adjuvant in clinical practice for seasonal diseases and the treatment of respiratory tract disorders.
Objective: This trial aims to verify the efficacy of the tested dietary supplement in a pediatric population with acute tonsillopharyngitis/rhinopharyngitis (ATR).
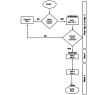
Methods: The trial includes children aged between 3 and 10 years with ATR ≤48 h, a negative rapid test for beta-hemolytic streptococcus or culture identification of nasal and/or pharyngeal exudates, and SARS-CoV-2 infection. The dietary supplement tested is an oral solution already on the market based on Pelagon P-70 (equivalent to Pelargonium sidoides d.e. 133.3 mg/100 ml), propolis, zinc, and honey. The product is administered at 5 ml 3 times a day for 6 days for children younger than 6 years and 10 ml 3 times a day for 6 days for children older than 6 years. The study design is open label, randomized, and controlled, with the tested dietary supplement plus standard of care (SoC) versus SoC alone. Patients are enrolled from 3 sites in Romania. The change in Tonsillitis Severity Score and number of treatment failures (using ibuprofen or high-dose paracetamol as rescue medication) are the primary end points. Based on the Tonsillitis Severity Score and the 2-sample comparison of the means formula with a 5% significance level, 80% power, and a minimally clinically important difference of 2 (SD 3.85) points, 120 patients are required. To account for potential screening failures and dropouts, we need to screen a population of approximately 150 children.
Results: Patient enrollment began on June 3, 2021 (first patient's first visit), and ended on August 12, 2021 (last patient's last visit). The data collection period was from June 3, 2021, to September 16, 2021. The study was funded in February 2023. Data analysis is currently ongoing (April 2024). We expect the results to be published in a peer-reviewed clinical journal in the third quarter of 2024 and presented at scientific meetings in the last quarter of 2024.
Conclusions: The data from this trial may help identify new adjuvant treatments for children with ATR when streptococcal infection is excluded by a negative rapid test, thereby avoiding unnecessary antibiotic administration.
Trial registration: ClinicalTrials.gov NCT04899401 https://clinicaltrials.gov/study/NCT04899401.
International registered report identifier (irrid): DERR1-10.2196/53703.
Keywords: Pelargonium; dietary supplements; nasopharyngitis; pharyngitis; propolis; severity score; tonsillitis; zinc.
©Fabio Cardinale, Dionisio Franco Barattini, Federica Sbrocca, Alessandro Centi, Greta Giuntini, Maria Morariu Bordea, Dorina Herteg, Serban Rosu, Cristian Radu Matei. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 31.05.2024.
Conflict of interest statement
Conflicts of Interest: DFB and FS are employed at Opera CRO, the contract research organization that managed the study. GG and AC are employed at Pediatrica Srl. FC, SR, MMB, DH, and CRM declare no conflicts of interest.
Figures
Similar articles
-
Different antibiotic treatments for group A streptococcal pharyngitis.Cochrane Database Syst Rev. 2023 Nov 15;11(11):CD004406. doi: 10.1002/14651858.CD004406.pub6. Cochrane Database Syst Rev. 2023. PMID: 37965935 Free PMC article.
-
Tonsillectomy or adenotonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis.Cochrane Database Syst Rev. 2014 Nov 19;2014(11):CD001802. doi: 10.1002/14651858.CD001802.pub3. Cochrane Database Syst Rev. 2014. PMID: 25407135 Free PMC article.
-
Calcium supplementation commencing before or early in pregnancy, or food fortification with calcium, for preventing hypertensive disorders of pregnancy.Cochrane Database Syst Rev. 2017 Sep 26;9(9):CD011192. doi: 10.1002/14651858.CD011192.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Sep 16;9:CD011192. doi: 10.1002/14651858.CD011192.pub3. PMID: 28949421 Free PMC article. Updated.
-
Paracetamol (acetaminophen) or non-steroidal anti-inflammatory drugs, alone or combined, for pain relief in acute otitis media in children.Cochrane Database Syst Rev. 2016 Dec 15;12(12):CD011534. doi: 10.1002/14651858.CD011534.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2023 Aug 18;8:CD011534. doi: 10.1002/14651858.CD011534.pub3. PMID: 27977844 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
The Effectiveness of a Dietary Supplement with Honey, Propolis, Pelargonium sidoides Extract, and Zinc in Children Affected by Acute Tonsillopharyngitis: An Open, Randomized, and Controlled Trial.Pharmaceuticals (Basel). 2024 Jun 19;17(6):804. doi: 10.3390/ph17060804. Pharmaceuticals (Basel). 2024. PMID: 38931472 Free PMC article.
-
Open, Randomised, Controlled Study to Evaluate the Role of a Dietary Supplement Containing Pelargonium sidoides Extract, Honey, Propolis, and Zinc as Adjuvant Treatment in Children with Acute Tonsillopharyngitis.Children (Basel). 2025 Mar 10;12(3):345. doi: 10.3390/children12030345. Children (Basel). 2025. PMID: 40150627 Free PMC article.
References
-
- Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010 Sep;126(3):e557–64. doi: 10.1542/peds.2009-2648. https://publications.aap.org/pediatrics/article-abstract/126/3/e557/6615... peds.2009-2648 - DOI - PubMed
-
- Windfuhr JP, Toepfner N, Steffen G, Waldfahrer F, Berner R. Clinical practice guideline: tonsillitis I. Diagnostics and nonsurgical management. Eur Arch Otorhinolaryngol. 2016 Apr;273(4):973–87. doi: 10.1007/s00405-015-3872-6. https://europepmc.org/abstract/MED/26755048 10.1007/s00405-015-3872-6 - DOI - PMC - PubMed
-
- ESCMID Sore Throat Guideline Group. Pelucchi C, Grigoryan L, Galeone C, Esposito S, Huovinen P, Little P, Verheij T. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012 Apr;18 Suppl 1:1–28. doi: 10.1111/j.1469-0691.2012.03766.x. https://linkinghub.elsevier.com/retrieve/pii/S1198-743X(14)61968-6 S1198-743X(14)61968-6 - DOI - PubMed
-
- Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013 Nov 05;2013(11):CD000023. doi: 10.1002/14651858.CD000023.pub4. https://europepmc.org/abstract/MED/24190439 - DOI - PMC - PubMed
-
- Linder JA, Bates DW, Lee Grace M, Finkelstein Jonathan A. Antibiotic treatment of children with sore throat. JAMA. 2005 Nov 09;294(18):2315–22. doi: 10.1001/jama.294.18.2315. https://jamanetwork.com/journals/jama/fullarticle/201839 294/18/2315 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous